The current state-of-the-art for H2 storage for alternative fuels is compressed H2 at 700 bar. This has all the risks associated with the storage and transport of high-pressure gases. Liquid hydrogen-storage materials are safer and have the potential to take advantage of the existing liquid-based distribution infrastructure such as pipelines, tankers, and retail outlets. This would make the transition to a H2-based energy economy cheaper and easier.
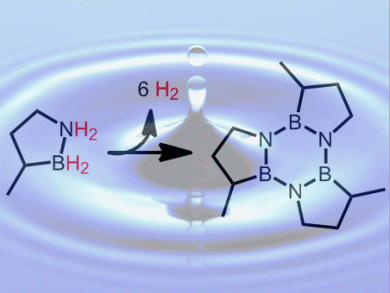
Shih-Yuan Liu and colleagues, University of Oregon, USA, have developed the boron and nitrogen containing heterocycle, BN-methylcyclopentane, as a H2 storage material. This is an air- and moisture-stable liquid at room temperature and pressure. It releases two equivalents of H2 per molecule (4.7 wt %) both thermally, at temperatures above 150 °C, and catalytically, at temperatures below 80 °C. A variety of cheap and abundant metal halides, including FeCl2, can be used as the catalysts. It forms a trimer upon H2 desorption (pictured) and can be converted back to the charged fuel in high yield under relatively mild conditions.
This system could be a viable candidate for liquid-phase hydrogen storage in mobile and carrier applications.
- A Single-Component Liquid-Phase Hydrogen Storage Material
W. Luo, P. G. Campbell, L. N. Zakharov, S.-Y. Liu,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja208834v