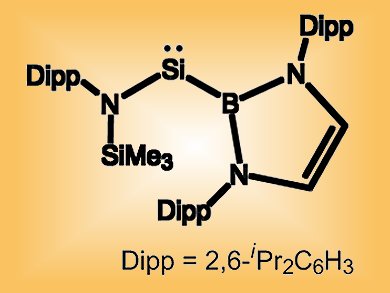
Sub-valent silicon compounds such as two-coordinate acyclic silylenes, SiR2, normally have a fleeting existence at around room temperature. Researchers at Oxford University, UK, and their colleagues at University College London, UK, and Monash University, Australia, have used the strong sigma-donor properties and high steric loading of their boron-based ligand to synthesize a two-coordinate acyclic silylene that is stable up to 130 °C.
The species undergoes oxidative addition reactions with dihydrogen as well as with alkyl C–H bonds. This, the researchers say, suggests a small singlet–triplet gap that hints at reactivity more characteristic of transition metal systems than organosilicon compounds.
- A Stable Two-Coordinate Acyclic Silylene,
A. V. Protchenko, K. Hassomal Birjkumar, D. Dange, A. D. Schwarz, D. Vidovic, C. Jones, N. Kaltsoyannis, P. Mountford, S. Aldridge,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja301042u