Researchers at the University of Groningen and DSM Pharmaceutical Products, both the Netherlands, have engineered the enzyme ethylaspartate ammonia lyase to allow them to carry out the asymmetric synthesis of unnatural amino acids. The enzyme usually transforms 3-methylaspartate into ammonia and 2-methylfumarate. The team has modified it to accept a variety of other substrates, including substituted amines and fumarates. They created two mutant versions both of which have a larger active site than normal. One accepts diverse linear and cyclic alkylamines and the other, fumarate derivatives with various substituents at the C-2 position. Both mutants retain the stereoselectivity of the original enzyme.
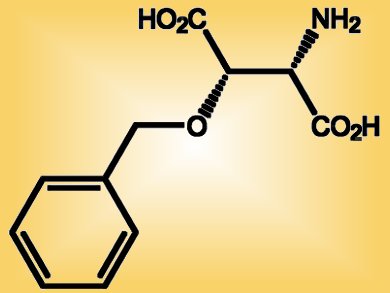
The synthesis was demonstrated with the highly enantio- and diastereoselective synthesis of an important inhibitor of neuronal excitatory glutamate transporters in the brain, threo-3-benzyloxyaspartate (pictured).
- Engineering methylaspartate ammonia lyase for the asymmetric synthesis of unnatural amino acids,
H. Raj, W. Szymanski, J. de Villiers, H. J. Rozeboom, V. Puthan Veetil, C. R. Reis, M. de Villiers, F. J. Dekker, S. de Wildeman, W. J. Quax, A.-M. W. H. Thunnissen, B. L. Feringa, D. B. Janssen, G. J. Poelarends,
Nature Chem. 2012.
DOI: 10.1038/nchem.1338