The direct, by-product–free conversion of basic feedstocks to products of medicinal and agricultural relevance is a broad goal of chemical research. Although many important methods for catalytic asymmetric carbon-carbon bond formation exist, much of this technology is not well suited for implementation on a large scale.
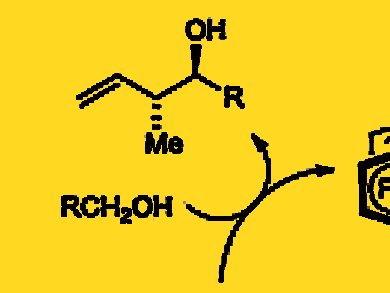
Michael J. Krische and colleagues, University of Texas, Austin, USA, report the direct redox-triggered carbon-carbon coupling of alcohols and butadiene with the use of a ruthenium catalyst modified by a chiral phosphate counterion. The direct coupling of alcohols and butadiene forms products of carbonyl crotylation with high levels of anti-diastereoselectivity and enantioselectivity in the absence of stoichiometric by-products.
As the researchers illustrate in the case of carbonyl crotylation, alcohol-butadiene hydrohydroxyalkylations enhance synthetic efficiency by removing the degrees of separation between reagent and feedstock, while bypassing discrete alcohol oxidation and the generation of stoichiometric byproducts.
These initial findings set the stage for the development of catalysts that exhibit enhanced stereoselectivities and substrate scope.
- Enantioselective C–H Crotylation of Primary Alcohols via Hydrohydroxyalkylation of Butadiene,
Jason R. Zbieg, Eiji Yamaguchi, Emma L. McInturff, Michael J. Krische,
Science 2012, 336, 324–327.
DOI: 10.1126/science.1219274