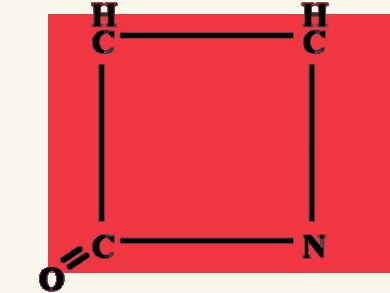
ß-lactams, a family of antibiotics whose activity depends on a ß-lactam ring (pictured), fight a wide spectrum of bacterial infections. Nevertheless, their therapeutic properties are hampered by ß-lactamases, bacterial hydrolases that inactivate the β-lactam ring and confer antibiotic resistance.
Javier González, Universidad Nacional de Rosario, Argentina, and colleagues have demonstrated that B1 Zn(II)-dependent ß-lactamases are crucially regulated by cysteine-Zn(II) interactions.
The in vivo activity of the enzyme is determined by Zn(II) availability. The presence of a cysteine residue in position 221 is essential to tune zinc binding affinity, guaranteeing an optimal metal uptake and, thus, the catalysis even at limiting metal concentrations.
By providing a new structural understanding of ß-lactamases’ activity, this study opens the doors for generating metallo ß-lactamase inhibitors targeting resistant bacteria.
- Metallo-β-lactamases withstand low Zn(II) conditions by tuning metal-ligand interactions,
J. M. González, M. R. Meini, P. E. Tomatis, F. J. Martín, J. A. Cricco, A. J. Vila.
Nat. Chem. Biol. 2012, 8 (8), 698-700
DOI: 10.1038/nchembio.1005