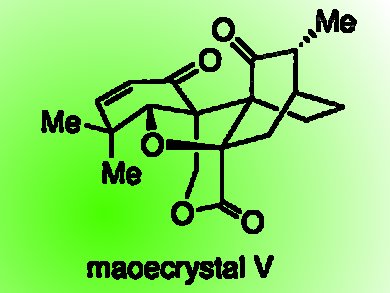
Maoecrystal V was first isolated in 1994 from the leaves of Isodon eriocalyx, a Chinese medicinal herb. It has considerable anticancer activity in vitro. It has a highly condensed pentacyclic structure, in which there are three contiguous quaternary carbon stereogenic centers. A successful synthesis of maoecrystal V could bring with it new compounds with therapeutically exploitable anticancer activity.
Feng Peng and Samuel J. Danishefsky, Columbia University, New York, USA, describe the total synthesis of racemic maoecrystal V.
Epoxides play a key role in the synthesis design. In the first instance, a stereospecific nucleophilic epoxidation reaction serves to install a C-8 oxygen functional group.
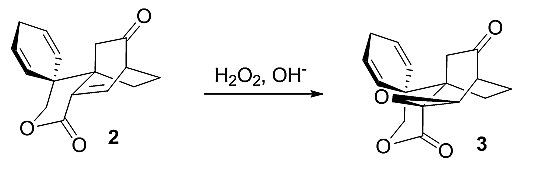
pictures show synthesis strategy on model compounds 2–7.
In a later step, a hydroxyl-directed epoxidation serves to differentiate the C1–C2 and C4–C5 olefins.
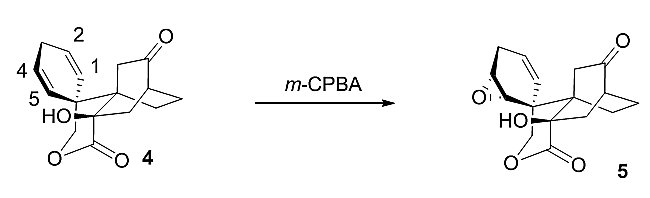
Subsequently, an epoxide rearrangement serves to install the required A:C trans-fusion. This reaction was designed to achieve delivery of a hydrogen to the hindered β-face of the tetrahydrofuran (C) ring system. The trick involved delivery of a hydrogen atom to the more hindered face by intramolecular means.
.jpg)
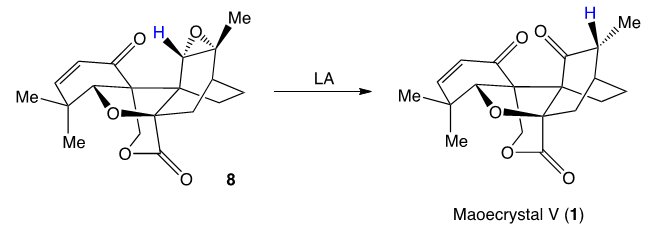
Finally, related logic was applied by the team for the last step of the synthesis: Again, an epoxide rearrangement served to deliver a hydrogen atom from the more hindered face of the molecule.
- Total Synthesis of (±)-Maoecrystal V,
Feng Peng and Samuel J. Danishefsky,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja309905j