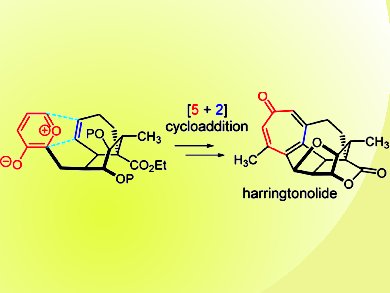
Harringtonolide (pictured), hainanolidol, and fortunolides A and B are representative members of Cephalotaxus norditerpenes. Harringtonolide and related natural products may have therapeutic potential as anticancer agents.
Weiping Tang and colleagues, University of Wisconsin, Madison, USA, developed a new synthetic route for hainanolidol and harringtonolide. The tetracyclic carbon skeleton of hainanolidol and harringtonolide was efficiently constructed by an intramolecular oxidopyrylium-based [5+2] cycloaddition. An anionic ring-opening strategy was developed for the cleavage of the ether bridge in 8-oxabicyclo[3.2.1]octenes derived from the [5+2] cycloaddition. Conversion of cycloheptadiene to tropone was realized by a sequential [4+2] cycloaddition, Kornblum−DeLaMare rearrangement, and double elimination.
This new synthetic route offers the flexibility to access other members of Cephalotaxus norditerpenes and various simplified analogues.
- Stereoselective Total Synthesis of Hainanolidol and Harringtonolide via Oxidopyrylium-Based [5 + 2] Cycloaddition,
Min Zhang, Na Liu, Weiping Tang,
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja406255j