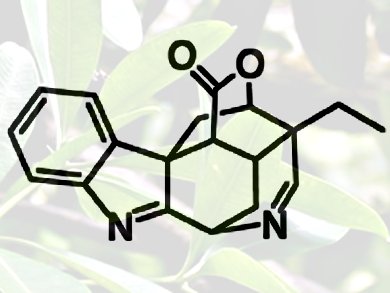
Monoterpenoid indole alkaloids have shown to act on a wide variety of physiological targets in the body. Some have been shown to have opioid activity, others cytotoxic behavior. Scholarisine A itself was first isolated from the plant Alstonia scholaris, which is used in traditional Chinese medicine to purportedly treat respiratory conditions. As such, finding ways to synthesize previously inaccessible skeletons could help build a new library of molecules for testing in the medicinal chemistry lab against various disease assays.
Myles Smith and Scott Snyder, Columbia University, New York, USA, started with commercial materials but used a unique C–H arylation reaction to build the multiply ringed polycyclic core of (+)-scholarisine A.
They completed the enantioselective synthesis of (+)-scholarisine A in 14 steps, beginning with a Diels−Alder reaction between a known dienophile and a methyl coumalate. The team exploited the general idea that the core is a carbocyclic substructure that they could produce with a flourish from relatively accessible oxabicyclo[2.2.2]octane intermediate.
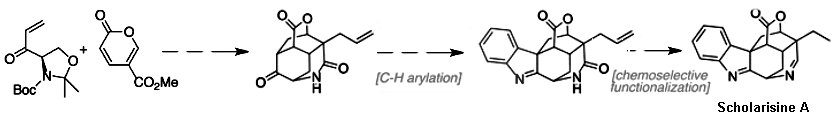
- A Concise Total Synthesis of (+)-Scholarisine A Empowered by a Unique C–H Arylation
Myles W. Smith, Scott A. Snyder
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja406546k