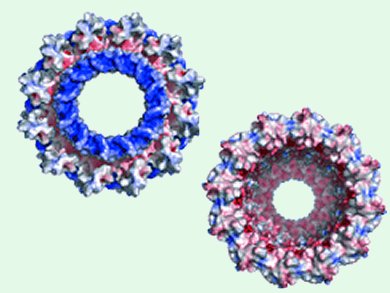
Biology offers much by way of nanoscopic “devices”, such as protein nanopores. However, exploiting them in non-biological applications, such as chemical analysis and medical diagnostics requires us to have much greater control over their characteristics than is offered by the native systems in their natural state.
Giovanni Maglia and colleagues at the University of Leuven, Belgium, have used the techniques of direct evolution to force the microbe Salmonella typhi to make versions of its nanopore protein Cytolysin A with specific pore sizes based on the 12mer (33 angstrom bore), 13mer (37 angstrom), and 14mer (42 angstrom) oligomeric forms of the protein. The team demonstrated that each analogue essentially has the same chemistry but the different pore sizes means that they can target different analytes, in this case other proteins, that neatly fit one pore but not the others.
- Tuning the Size and Properties of ClyA Nanopores Assisted by Directed Evolution,
Misha Soskine, Annemie Biesemans, Marc De Maeyer, Giovanni Maglia,
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja4053398