Current cathode materials suffer from capacity limitations. Therefore, battery research is looking for new cathode materials. Sheng-Heng Chung and Arumugam Manthiram, University of Texas, Austin, TX, USA, present an eggshell membrane recycled from domestic waste as cathode to improve the performance of Li-S batteries.
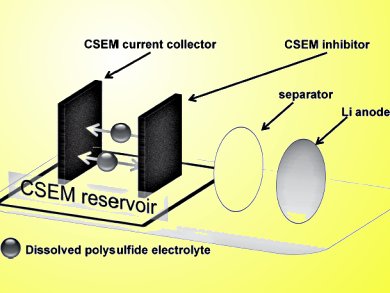
The eggshell membrane has a uniform microporous structure and an interwoven fiber network. This makes it an excellent nanomaterial synthesis template. Free-standing carbonized sucrose-coated eggshell membranes (CSEMs) form a reservoir for the dissolved Li2S6 polysulfide catholyte (pictured).
Carbonized eggshell membranes (CEMs) are not as conductive as a metal, but the carbonized sucrose improves the electrical conductivity without reducing the microporosity. The CSEM reservoir has one CSEM as a current collector, another CSEM as an inhibitor, and the dissolved polysulfide catholyte stabilized in between. This configuration provides Li/dissolved polysulfide cells.
These show high discharge capacity (1327 mA h g− ), excellent cycle stability (over 100 cycles), and high sulfur loading (3.2 mg cm−2). Moreover, the free-standing CSEM itself has high electrical conductivity and a hierarchical macro/microporous structure.
- Carbonized Eggshell Membrane as a Natural Polysulfide Reservoir for Highly Reversible Li-S Batteries,
Sheng-Heng Chung, Arumugam Manthiram,
Adv. Mater. 2013.
DOI: 10.1002/adma.201304365