Multiple electrochromic materials are used in devices such as smart windows or electronic paper. Electrochromism is a process by which the color of a material is changed through an electrochemical reaction; this process is reversible. Increasing the number of colors is achieved by using an electrochrome deposited on a working electrode for each color. As the same number of working electrodes as colors is required, full-color devices are complicated to assemble.
Jun Matsui and colleagues from Yamagata and Tohoku Universities, Japan, have reported a new strategy that allows the use of one electrode, thus significantly simplifying the construction of such devices. Instead of a typical bilayer electrode, they used a three layer electrode.
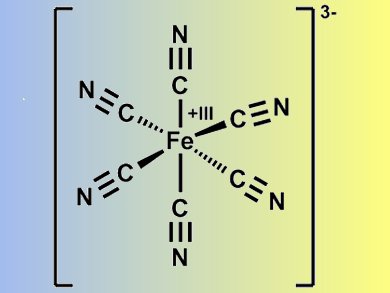
On top of a conducting electrode, in this case ITO (indium tin oxide), a first layer of Prussian Blue (PB; KFeIII[FeII(CN)6] nanoparticles, a second nanosheet layer containing the ruthenium complex p(DDA/Rubpy32+, and a third PB layer is placed.
Prussian Blue (pictured) is a well-known and soluble electrochromic material. Its color changes from blue to yellow when oxidized to become Prussian Yellow (PY: KFeIII[FeIII(CN)6]). The reduced form is Prussian white and is transparent (PW: KFeII[FeII(CN)6]). The researchers found that PB and PY can exist simultaneously. Therefore, this ultrathin trilayer film allows combinations of the following colors to be made: blue and orange; yellow and yellow; and blue, yellow, and orange.
The current limitation is the film thickness, which is so thin that color changes cannot be observed by the naked eye. Future efforts are now focused on increasing the thickness.
- A Trilayer Film Approach to Multicolor Electrochromism,
Jun Matsui, Rie Kikuchi, Tokuji Miyashita,
J. Am. Chem. Soc. 2014.
DOI: 10.1021/ja41077861