Heterocycles are important structures in organic chemistry and appear widely in the pharmaceutical, agrochemical, and fine chemical industries. Although there are a number of ways of synthesizing heterocycles, there are few examples that use an approach called direct imine acylation (DIA). DIA is a method that couples an imine functional group (–C=N–) with a carboxylic acid (–CO2H).
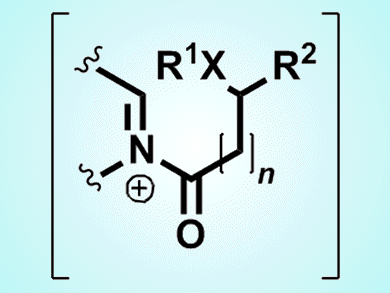
Richard Taylor and colleagues, University of York, UK, realized that by using DIA with carboxylic acids that also contain either a nucleophile (oxygen, nitrogen, sulfur), or a carbon pro-nucleophile (active methylene, aromatic group, alkene), a wide variety of heterocyclic products could be produced, including more complex ring structures. The reaction between the imine and carboxylic acid with propylphosphonic acid anhydride and diisopropylethylamine forms an positively charged intermediate (N-acyliminium ion; see picture), which goes on to react with whichever nucleophile or pro-nucleophile is present on the carboxylic acid reagent to form the final product. These reactions, which work particularly well with sulfur, give good yields (typically 65–97 %) and work to develop an asymmetric DIA method is currently underway.
- Direct Imine Acylation for Molecular Diversity in Heterocyclic Synthesis,
William P. Unsworth, Graeme Coulthard, Christiana Kitsiou, Richard J. K. Taylor,
J. Org. Chem. 2014.
DOI: 10.1021/jo402768r