The development of solar panel materials that are both non-toxic and made from readily available elements rather than rare and precious metals is a priority in developing a sustainable technology. To this end, sulfide materials containing copper, tin, and zinc, so called kesterites, have been proposed as solar cell absorber materials. Experimental solar cells using Cu2ZnSnS4 (CZTS) have demonstrated energy conversion efficiencies of 8.4 % and 12 % for a seleno-sulfide analogue.
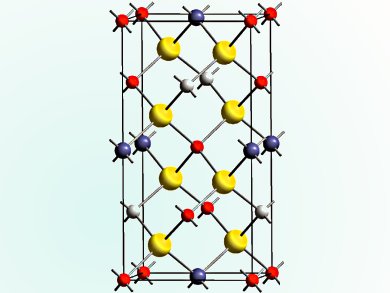
Cu/Zn disorder in the kesterite Cu2ZnSnS4 derivatives is an important issue for photovoltaic performances. Unfortunately, Cu+ and Zn2+ have very close atomic scattering factors, which makes it impossible to distinguish the ordered kesterite and the disordered kesterite structures from conventional X-ray diffraction experiments, even on single crystals.
Alain Lafond and his colleagues, Nantes University, and Pierre Fertey, Soleil Synchrotron, Saint-Aubin, both France, have demonstrated that it is possible to carry out resonant diffraction of a single crystal of the semiconductor CZTS.
The powdered precursor was prepared using a ceramic synthesis at 1023 K from the corresponding elements Cu, Zn, Sn and S. The product is heated for a further 96 hours to anneal it before it is plunged into ice-water to lock in the chemical structure present at that elevated temperature, a process known as quenching.
Laboratory powder X-ray diffraction and energy-dispersive X-ray spectroscopy analyses were used to test the purity of tiny single crystals picked out of the powder. Then high-performance resonant diffraction on the CRISTAL beamline of the Soleil synchrotron was carried out. The data obtained showed the annealing process generates a disordered structure that can be distinguished from the ordered kesterite structure despite the otherwise similar X-ray scattering pattern that would be generated by the copper and zinc ions in the ordered form.
This technique is demonstrated to be sensitive enough to clearly distinguish between copper and zinc, and to open the door to an accurate description of the cationic distribution within the kesterite structure.