N–H bonds are common in natural and synthetic organic compounds and considerable interest has recently been focused on the design of tandem or sequential reactions for the efficient construction of complex molecules by insertion of functional groups into the N–H bond. Amongst these reactions, the lanthanide-catalyzed sequential insertion of alkenes (alkynes) and other polar unsaturated functional groups into the N–H bond has not yet been fully explored; this can be attributed to the difficulties encountered in controlling required selectivity and to the incompatibility of inserting of polar functional groups into non-polar groups. In addition, heteroatoms often coordinate more strongly to rare earth metals than alkenes (alkynes), inhibiting catalytic turnover.
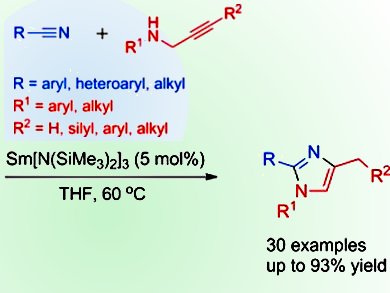
X. Zhou and colleagues, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials and the State Key Laboratory of Organometallic Chemistry, China, have sought to overcome these difficulties by developing a Ln[N(SiMe3)2]3-catalyzed cyclization reaction of a range of nitriles with propargylamines to form 1,2,4-trisubstituted imidazoles. Both the nitriles and propargylamines are readily available and the reactions proceed by an efficient, highly regioselective insertion of C≡N and C≡C into the N–H bond. The trisubstituted imidazoles produced are generally difficult to synthesize by other means, making this reaction of high interest to synthetic organic chemists.
- Ln[N(SiMe3)2]3-Catalyzed Cross-Diinsertion of C≡N/C≡C into an N–H Bond: Facile Synthesis of 1,2,4-Trisubstituted Imidazoles from Propargylamines and Nitriles,
Longcheng Hong, Yinlin Shao, Lixin Zhang, Xigeng Zhou,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402701