Organic peroxides and related peroxy compounds are among the most accessible oxidants that mimic the environmentally friendly characteristics of natural oxidants. However, many peroxy compounds have limited applications and they are often dangerous to use.
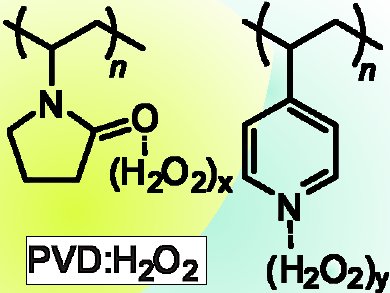
G. K. Surya Prakash, Thomas Mathew, George A. Olah, and colleagues, University of Southern California, Los Angeles, CA, USA, have prepared complexes of poly(N-vinylpyrrolidone) (PVD) and poly(4-vinylpyridine) (PVP) with hydrogen peroxide (pictured) which show impressive promise as efficient solid forms of H2O2 for more oxidation studies.
The team has studied their synthetic utility as solid H2O2 equivalents for the selective oxidation of sulfides to sulfoxides and ketones to gem-dihydroperoxides. Handling of the oxidant H2O2 was much safer and more convenient and the amount of side products was reduced. Conversion of aryl alkyl sulfides to their corresponding sulfoxides as well as the preparation of the gemdihydroperoxy compounds from corresponding ketones were achieved in good to excellent yields.
PVD–H2O2 performs significantly better than PVP–H2O2 in most applications. According to the researchers, this might be simply a difference in H2O2 loading.
Moreover, the complexes are more environmentally friendly than other oxidants as water is the by-product and they can be recycled and reused either with the remaining amount of H2O2 or enhancing the H2O2 content by additional complexation depending on the amount required.
- Poly(N-vinylpyrrolidone)–H2O2 and poly(4-vinylpyridine)–H2O2 complexes: solid H2O2 equivalents for selective oxidation of sulfides to sulfoxides and ketones to gem-dihydroperoxides,
G. K. Surya Prakash, Anton Shakhmin, Kevin E. Glinton, Sneha Rao, Thomas Mathew, George A. Olah,
Green Chem. 2014, 16, 3616.
DOI: 10.1039/c4gc00586d