The success of electric vehicles requires the development of safe, environmentally friendly, rechargeable batteries with a high energy density. Recently developed lithium sulfur batteries meet all these criteria due to their high energy density. However, limited lithium resources as well as unresolved safety issues hinder a commercial usage.
Magnesium is a promising alternative, as it overcomes the previously mentioned drawbacks and has a high theoretical energy density with a sulfur cathode. The problem here is to find a suitable electrolyte for sulfur magnesium (S/Mg) batteries, that is stable with the electrode materials and has a wide electrochemical window.
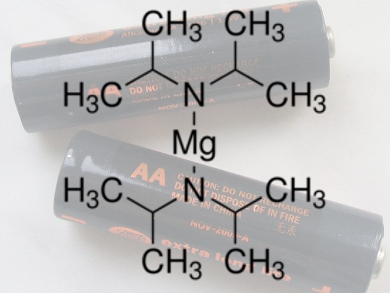
Maximilian Fichtner, Karlsruhe Institute of Technology (KIT), Karlsruhe, Germany, and colleagues used hexamethyldisilazide (HMDS)2Mg-based diglyme and tetraglyme (HMDS = hexamethyldisilazide) as non-nucleophilic electrolyte solutions with an ionic liquid as an additive. This combination shows beneficial effects to the electrochemical conversion between Mg and S in Mg/S batteries and a high discharge potential of about 1.65 V was recorded for the first time.
However, a large hysteresis between charge and discharge is revealed, which indicates a quick fading in capacity and makes further improvement on Mg/S batteries necessary.
- Performance Improvement of Magnesium Sulfur Batteries with Modified Non-Nucleophilic Electrolytes
Zhirong Zhao-Karger, Xiangyu Zhao, Di Wang, Thomas Diemant, R. Jürgen Behm, Maximilian Fichtner
Adv. Energy Mater. 2014.
DOI: 10.1002/aenm.201401155