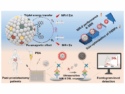
Jun Jin and Jiantai Ma, Lanzhou University, China, and colleagues, developed a supermagnetic nanocomposite with good selectivity, high saturation magnetization, and good water dispersibility that clears Cd2+ from human blood. Cadmium is a key component in battery production, present in pigments, coatings, and commonly used in electroplating. Cd2+ binds to proteins and damages organs; it is carcinogenic.
The nanocomposite, PAD-PEG-Fe3O4@PEI, was built up from four components: Magnetic iron oxide nanoparticles, chosen for their low toxicity, are coated with polyethylenimine (PEI), which binds to Cd2+. The coating also reduces the chances of nanoparticle uptake by red blood cells, maximising their circulation time in the blood. Polyethylene glycol (PEG) was grafted onto this as an anchor for negatively charged 2,2′-(phenylazanediyl) diacetic acid (PAD), which counteracts interactions between the nanoparticles and plasma proteins or white blood cells. The average particle size is about 50 nm.
80 % of Cd2+ at a concentration of 1 ppm in a 1 mL blood sample were removed with good selectivity when compared to other positively charged ions, such as Ca2+ and Zn2+.
The idea is that the nanocomposites would be injected into a vein, bind to cadmium ions in the blood, and be removed by circulating the blood through a magnetic field to attract the magnetic nanocomposite-cadmium ion complexes. The detoxified blood would then be returned to the body.
Mansoor Amiji, Northeastern University, USA, as stated in an article of Chemistry World, points out that PEI is toxic to cells and positively charged PEI can attract any negatively charged ion. Future work should show that the nanoparticles can actually work in a biologically relevant system.
- 2, 2′-(phenylazanediyl) diacetic acid modified Fe3O4@PEI for selective removal of cadmium ions from blood,
Jun Jin, Fang Yang, Fengwei Zhang, Wuquan Hu, Shao-bo Sun, Jiantai Ma,
Nanoscale 2012.
DOI: 10.1039/C2NR11481J