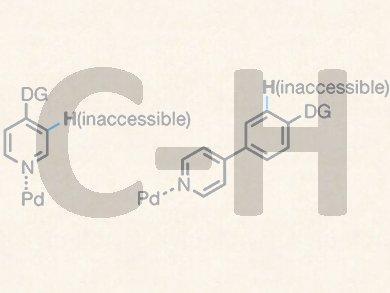
Heterocycles are essential building blocks in medicinal chemistry and pharmaceutics. However, heteroatoms, such as nitrogen and sulphur atoms, may severely interfere with directed C−H activation of heterocyles through coordination to catalytically active metals.
To overcome such limitations, Jin-Quan Yu, Hui-Xiong Dai and co-workers at Shanghai Institute of Organic Chemistry, Shanghai, China and The Scripps Research Institute, Lo Jolla, CA, USA, have introduced a simple N-methoxy amide group to heterocyclic substrates as both a directing group and an anionic ligand. In the presence of Pd catalysts and t-butylisocyanide, the substrates are exclusively ortho-functionalized into 3-(imino)isoindolinones, which can then be converted into useful intermediates such as lactams and diesters.
This strategy, while providing excellent regioselectivity, has elegantly solved the complications presented by heteroatoms. Its scope may also be expanded to include other catalytic C−H functionalizations.
- Overcoming the limitations of directed C–H functionalizations of heterocycles
Yue-Jin Liu, Hui Xu, Wei-Jun Kong, Ming Shang, Hui-Xiong Dai, Jin-Quan Yu
Nature 2014, 515, 389–393.
DOI: 10.1038/nature13885