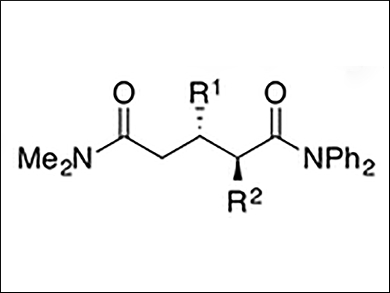
Shu̅ Kobayashi and colleagues, The University of Tokyo, Japan, have performed the first example of a highly enantioselective catalytic direct-type reaction of simple amides. In an asymmetric direct 1,4-addition, simple amides without any activating group reacted with α,β-unsaturated carbonyl compounds at the α-position. A catalytic amount of a novel chiral catalyst consisting of a potassium base and the macrocyclic chiral crown ether binaphtho-34-crown-10 was used.
The desired 1,5-dicarbonyl compounds (pictured) were obtained in high yields with excellent diastereo- and enantioselectivities.
- Catalytic Asymmetric Direct-Type 1,4-Addition Reactions of Simple Amides,
Hirotsugu Suzuki, Io Sato, Yasuhiro Yamashita, Shu̅ Kobayashi,
J. Am. Chem. Soc. 2015.
DOI: 10.1021/jacs.5b01943