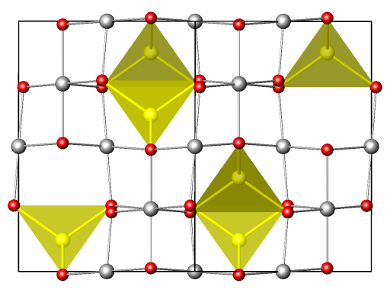
Lithium-ion batteries dominate the market for mobile devices, and are used in electric vehicles. They are, however, limited in their energy storage capacity, and the search for improved electrode materials is ongoing. Replacing lithium with multivalent ions such as magnesium could increase capacity by doubling the charge per intercalated ion.
Jordi Cabana, University of Illinois at Chicago, USA, and co-workers could show that magnesium intercalates reversibly into the tetrahedral sites of spinel-type Mn2O4. The team delithiated LiMn2O4 using hydrochloric acid solution and electrochemically intercalated the magnesium ions. They characterized the resulting MgMn2O4 extensively, using atomic resolution X-ray spectroscopy, bulk X-ray diffraction, scanning transmission electron microscopy (STEM), and 25Mg NMR spectroscopy.
The material has a theoretical capacity of 270 mAh/g, which coupled with elemental magnesium would surpass the energy density of all commercially available technologies. While the electrochemical cells used in this work do not yet consitute a usable magnesium-ion battery, it shows that intercalation of multivalent ions into spinels is a promising approach for high-voltage, high-capacity cathode materials.
- Direct Observation of Reversible Magnesium Ion Intercalation into a Spinel Oxide Host,
Chunjoong Kim, Patrick J. Phillips, Baris Key, Tanghong Yi, Dennis Nordlund, Young-Sang Yu, Ryan D. Bayliss, Sang-Don Han, Meinan He, Zhengcheng Zhang, Anthony K. Burrell, Robert F. Klie, Jordi Cabana,
Adv. Mater. 2015.
DOI: 10.1002/adma.201500083