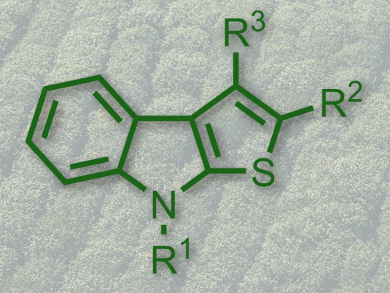
Roman A. Irgashev, Ural Division of the Russian Academy of Sciences, Ekaterinburg, Russia, and colleagues have developed a short and robust synthesis of 2-(hetero)aryl substituted thieno[2,3-b]indoles (pictured) from easily available 1-alkylisatins and acetylated (hetero)arenes.
The two-step approach includes the “aldol-crotonic” type condensation of N-alkylated isatins with acetophenones or their heterocyclic analogues. This is followed by treatment of the intermediate 3-(2-oxo-2-(hetero)arylethylidene)indolin-2-ones with Lawesson’s reagent. The latter process involves two sequential reactions, namely the reduction of the C=C ethylidene double bond of the intermediate indolin-2-ones, followed by a Paal–Knorr cyclization.
According to the researchers, this approach provides easy access to compounds of the electron-rich thieno[2,3-b]indole family. They are seen as promising building-blocks for the development of new photo- and electrosensitive molecules, e.g., novel push-pull dyes for dye-sensitized solar cells.
- A new and convenient synthetic way to 2-substituted thieno[2,3-b]indoles,
Roman A. Irgashev, Arseny A. Karmatsky, Gennady L. Rusinov, Valery N. Charushin,
Beilstein J. Org. Chem. 2015, 11, 1000–1007.
DOI: 10.3762/bjoc.11.112