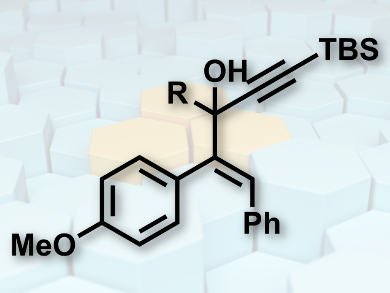
Christine Basmadjian, Fan Zhang, and Laurent Désaubry, University of Strasbourg, CNRS, Illkirch, France, have investigated dehydration and acid-cyclization reactions of 1-styrylpropargyl alcohols (pictured). Basis of their research was the synthesis of cyclopentenone from 1-styrylpropargyl alcohols using a molybdenum(VI)-catalyzed etherification of allylic alcohol and a gold(I)-catalyzed intramolecular cyclization process. Serendipitously, the team discovered a direct conversion of the 1-styrylpropargyl alcohols to cyclopentenone by using a Re2O7 catalyst.
Numerous compounds were synthesized in this manner with different 1-styrylpropargyl alcohols. These include a furan (3-(4-Methoxyphenyl)-5-methyl-2-phenylfuran), an acyclic enone ((E)–3-((E)-1-(4-Methoxyphenyl)-2-phenylvinyl)hept-3-en-2-one), and a naphthalenone (4-(4-Methoxyphenyl)-2-methyl-4-phenylnaphthalen-1(4H)-one). The researchers explain the diversity of structural motifs, especially the formation of the naphthaleone, by new carbocationic rearrangements of the 1-styrylpropargyl alcohols. They say minor substrate changes result in several mechanistic pathways.
- Novel carbocationic rearrangements of 1-styrylpropargyl alcohols,
Christine Basmadjian, Fan Zhang, Laurent Désaubry,
Beilstein J. Org. Chem. 2015, 11, 1017–1022.
DOI: 10.3762/bjoc.11.114