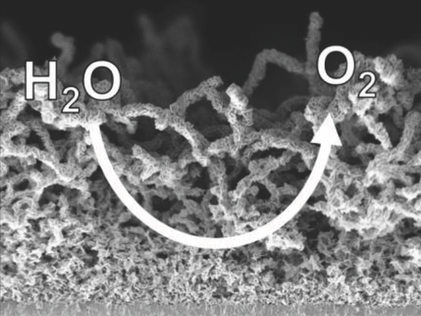
Efficient energy storage methods are important for renewables with fluctuating output such as wind and solar energy. Electrolyzing water and storing the energy as hydrogen is a well-investigated approach for this issue. However, splitting water electrochemically is often hampered by the high overpotential of the oxygen evolution reaction (OER) at the anode. Nickel-based materials with an intricate nanostructure and a high catalytic surface area could be a cost-effective and well-perfoming anode material. The enhancing effect of the nanostructure is, however, often diminished when such materials are incorporated into polymer-based binders to construct the final electrode.
Stefan Seeger and Georg R. Meseck, University of Zurich, Switzerland, and Emiliana Fabbri and Thomas J. Schmidt, Paul Scherrer Institut, Villigen, Switzerland, have developed silicone nanofilaments (SNFs) as a free-standing support for electrode materials. This development allows the production of binder-less nanostructured nickel oxide anodes for water splitting. The team used an electrode based on fluorine-doped tin oxide (FTO) as a current collector, which they coated with a carpet of SNFs (about 10 μm thick) by chemical vapor deposition. They immersed the substrate in nickel nitrate solution, which coated the nanofilaments with nickel hydroxide, and then calcined the samples at 400 °C to convert the hydroxide to nickel oxide.
The researchers compared the resulting electrode to an unsupported electrode and studied the oxygen evolution reaction using cyclovoltammetry. The supported electrode showed a significantly reduced overpotential at constant mass current density. This improvement in performance results in a three times higher mass current density at fixed overpotential. SNFs could thus be a viable strategy to build electrodes for water splitting, and the team is currently investigating doped oxides to improve the catalytic performance and lower the overpotential.
- Silicone Nanofilament Supported Nickel Oxide: A New Concept for Oxygen Evolution Catalysts in Water Electrolyzers,
Georg R. Meseck, Emiliana Fabbri, Thomas J. Schmidt, Stefan Seeger,
Adv. Mater. Interfaces 2015.
DOI: 10.1002/admi.201500216