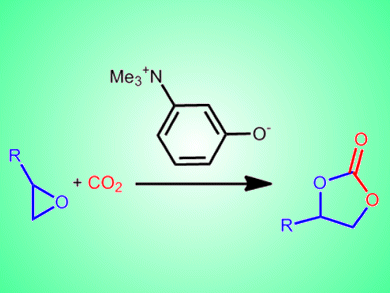
The chemical activation of carbon dioxide could not only help reduce CO2 levels in the atmosphere, but could provide a renewable feedstock for the chemical industry. The strong C=O bonds, however, make the activation and fixation of CO2 through organic syntheses difficult.
Takashi Sakai and co-workers, Okayama University, Japan, report a bifunctional organocatalyst based on an ammonium betaine framework for the cooperative activation of CO2 with epoxides. The team tested a range of catalysts in which the distance between the two functional groups of the betaine framework was varied. The conversion of 1,2-epoxyhexane to the cyclic carbonate was achieved with yields of >99 % with 1 mol% of the best catalyst, 3-(trimethylammonio)phenolate.
The betaine-CO2 adduct was shown to form readily at 15 °C and this catalytic system is currently one of the most active under metal-free, halogen-free, and solvent-free conditions.
- Bifunctional Organocatalyst for Activation of Carbon Dioxide and Epoxide To Produce Cyclic Carbonate: Betaine as a New Catalytic Motif
Y. Tsutsumi, K. Yamakawa, M. Yoshida, T. Ema, T. Sakai,
Org. Lett. 2010, 12.
DOI: 10.1021/ol102539x