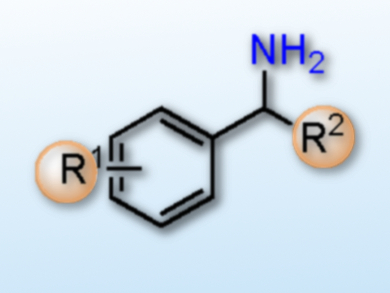
Primary amines are an important target for synthesis, both as products and as intermediates, for example in fine chemicals and drug production. While alcohol amination is a well-developed process to generate primary amines, their synthesis using reductive amination of ketones or aldehydes still has issues such as low yields and selectivities. A common problem is the undesired hydrogenation of the ketone to the corresponding alcohol.
Thomas Schaub, BASF SE, Ludwigshafen, Germany, and colleagues have developed an efficient and highly selective reductive amination of acetophenone derivatives to primary amines, using ammonia and hydrogen, as well as a ruthenium-based catalyst. The process uses [Ru(CO)ClH(PPh3)3] as a precatalyst in combination with diphosphine ligands. The simple 1,2-bis(diphenylphosphino)ethane (dppe) showed the best performance. Al(OTf)3 was used as an acidic co-catalyst.
The team used their optimized protocol on a range of acetophenone derivatives. The reaction proceeded with high selectivity, good to excellent yields, and good functional group tolerance. The researchers ascribe the high selectivity to the aluminium triflate co-catalyst, presumably caused by imine activation.
- Direct Synthesis of Primary Amines via Ruthenium-Catalysed Amination of Ketones with Ammonia and Hydrogen,
Joan Gallardo-Donaire, Martin Ernst, Oliver Trapp, Thomas Schaub,
Adv. Synth. Catal. 2016.
DOI: 10.1002/adsc.201500968