Producing adjustable potable mineral water from deep sea water (DSW) becomes more and more prevailing because it is pure, free of land pollutants, and rich in vital mineral ions, such as magnesium and calcium. Its application, however, is limited considerably by the high concentration of sulfate ions in DSW. This problem may be solvable through adopting a membrane separation process.
Adopting a laboratory-scaled electrodialysis (ED) process, Sung-Hwa Lin, National Ilan University, Yilan City, Taiwan, and his team have investigated the performance of a monovalent anion exchange permselective membrane in the reduction of the concentration of sulfate ions during the production of mineral source water from DSW. The dependence of the separation efficiency of anions on the operating time and the applied DC voltage was investigated based on a brine having salinity of about 15 % prepared from DSW.
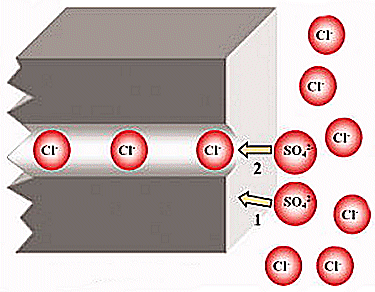
The experimental results reveal that if the applied DC voltage is high, the change in the liquid volume during ED is dominated by the ions transported and the effect of electroosmosis. In addition, the amount of Cl− transported correlates roughly linearly with the operating time, and the transport of sulfate ions is found to be blocked by Cl−, presumably because the pore size of the permselective layer is close to the size of sulfate ions.
Based on the constraints of high Cl− output, low SO42− content, and low electrical energy consumption, a region representing the possible optimal operation conditions for the ED is constructed.
- Preparation of mineral source water from deep sea water: Reduction of sulfate ion using selemion ASV membrane,
Hsiang-Yung Lu, Chih-Shan Lin, Shih-Chi Lee, Ming-Hong Ku, Jyh-Ping Hsu, Shiojenn Tseng, Sung-Hwa Lin,
AIChE J. 2010.
DOI: 10.1002/aic.12319