N-heterocyclic carbenes (NHCs) are very common and versatile ligands with tunable steric and electronic properties. In contrast to many other transition metals, there are very few examples of tantalum and niobium NHC complexes, especially with monodentate NHC ligands.
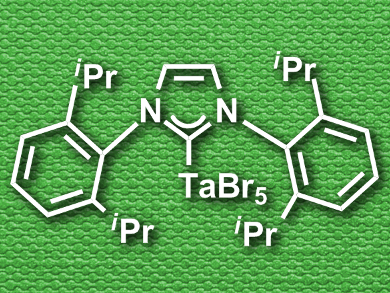
Fabio Marchetti, University of Pisa, Italy, and colleagues have synthesized and characterized the monodentate NHC complexes NbX5(Ipr) and TaY5(Ipr) (Ipr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene; X = F, Br; Y = F, Cl, Br). The researchers combined the metal pentahalides with the NHC ligand in toluene at around 80 °C and the complexes were formed in yields between 47 % and 64 %.
The complexes were characterized by elemental analysis and IR spectroscopy, as well as 1H, 13C, 19F and 93Nb NMR spectroscopy (where applicable). The team was also able to characterize the complex TaBr5(Ipr) using X-ray crystallography, the first crystallographic characterization of a tantalum complex with a monodentate NHC ligand. The researchers used density functional theory (DFT) calculations to gain insight into the bonding situation in the complexes, and found σ-type bonding between the metal and the NHC carbon atom with no meaningful π-interaction.
- Coordination complexes of niobium and tantalum pentahalides with a bulky NHC ligand,
Marco Bortoluzzi, Eleonora Ferretti, Fabio Marchetti, Guido Pampaloni, Stefano Zacchini,
Dalton Trans. 2016.
DOI: 10.1039/C6DT00533K