Sodium-ion batteries (SIBs) are a promising energy storage alternative to lithium-ion batteries due to the high abundance and low cost of sodium. A major challenge in the development of SIBs is the lack of efficient anodes that can store significant amounts of sodium ions in a reversible and rapid way.
One promising anode material is sodium titanate (Na2Ti3O7), whose structure consists of zigzag layers of titanium oxygen octahedra, and in which up to 3.5 sodium ions per formula unit can be intercalated. However, Na2Ti3O7 suffers from sluggish Na insertion and extraction kinetics as well as low cycling stability.
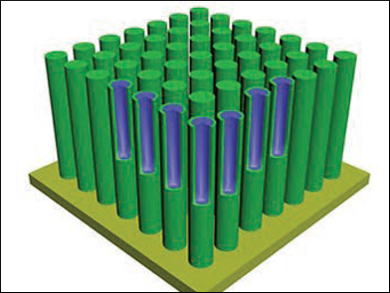
Liang Li, Soochow University, Suzhou, China, Yan Yu, University of Science and Technology of China, Hefei, and colleagues developed surface-engineered Na2Ti3O7 nanotubes on a titanium metal foil as an improved anode material. The nanotube arrays were synthesized using hydrothermal methods, and a TiO2 layer was then deposited on them using atomic layered deposition (ALD). The researchers further treated the nanotubes with sulfur vapor. During the sulfidation process, the titanium dioxide is doped by sulfur and becomes highly conductive.
The resulting array consists of periodic nanotubes of 3 µm in length and 30–40 nm in diameter. This SIB anode shows excellent sodium-ion storage stability enabled by the TiO2 coating and high rate capacities enabled by the sulfidation, which could be retained over 10000 cycles.
- Superior Sodium Storage in Na2Ti3O7 Nanotube Arrays through Surface Engineering,
Jiangfeng Ni, Shidong Fu, Chao Wu, Yang Zhao, Joachim Maier, Yan Yu, Liang Li,
Adv. Energy Mater. 2016.
DOI: 10.1002/aenm.201502568