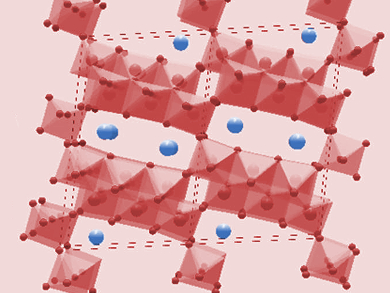
Sodium-ion batteries (NIBs) are possible cheaper alternatives to the commonly used lithium-ion batteries because of the high abundance and relatively low price of sodium. However, since Na ions are larger than Li ions, their diffusion into and out of anodes and the associated volume changes of the material can be problematic.
Lifang Jiao, Nankai University, Tianjin, China, and colleagues have developed an anode material for NIBs that consists of Na2Ti6O13 nanorods. The team prepared TiO2 particles by hydrolysis of tetrabutyl titanate and treated them with NaOH to give hydrated sodium titanate nanowires. They were annealed at 700 °C for 10 h to give the final nanorod material.
Na2Ti6O13 has a tunnel structure with diameters larger than Na ions, which facilitates diffusion. The framework is very stable against volume changes, which improves cycling stability. The nanostructure allows high rate capability because of improved access to the interior of the crystal for the Na ions. The nanorods showed a capacity of 172 mAh/g at 0.1 A/g and 109 mAh/g after 2800 cycles at 1 A/g. According to the researchers, this performance makes them a promising material for large-scale energy storage systems.
- Na2Ti6O13 Nanorods with Dominant Large Interlayer Spacing Exposed Facet for High-Performance Na-Ion Batteries,
Kangzhe Cao, Lifang Jiao, Wei Kong Pang, Huiqiao Liu, Tengfei Zhou, Zaiping Guo, Yijing Wang, Huatang Yuan,
Small 2016.
DOI: 10.1002/smll.201600845