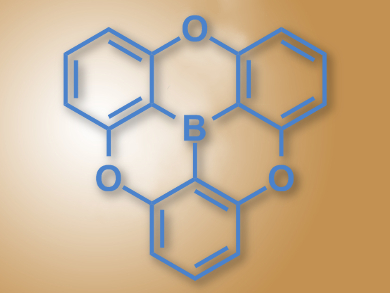
Triarylboranes are excellent electron acceptors due to their empty p-orbital and π-conjugation. They can be used, for example, as materials for organic light-emitting diodes (OLEDs) or as organic semiconductors. However, they are unstable in the presence of nucleophiles and at higher temperatures. Including electron-rich elements such as O or N close to the boron can stabilize the compounds, but lowers their ability to accept electrons.
Yuichi Kitamoto, Shuichi Oi, Tohoku University, Sendai, Japan, and colleagues have synthesized the first oxygen-bridged planar triphenylborane (pictured). The team started from resorcinol and 3-iodoanisole, synthesized the corresponding ether, included the central boron atom using n-butyl lithium, and in a final step closed the ring with an SNAr reaction.
The researchers characterized the compound using X-ray crystallography and found that the boron atom has the intended planar surroundings and the shortest C–B bonds among reported triarylboranes. The borane exhibits violet fluorescence. The compound has good electron-accepting properties due to its planar conjugated structure, but is stabilized by the oxygen bridges. According to the team, it could find uses as an organic electronic material.
- First Synthesis and X-ray Crystallographic Analysis of an Oxygen-bridged Planarized Triphenylborane,
Yuichi Kitamoto, Takatsugu Suzuki, Yasuo Miyata, Hiroshi Kita, Kenji Funaki, Shuichi Oi,
Chem. Commun. 2016.
DOI: 10.1039/C6CC02440H