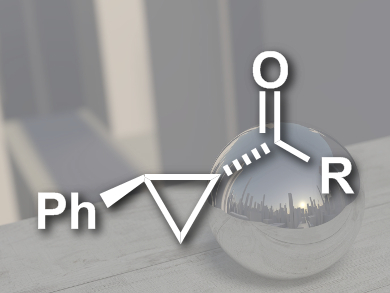
Chiral cyclopropanes are useful synthetic intermediates and a structural motif that can be found in many biologically active compounds. The cyclopropanation of olefins with diazoacetates is well established. Diazoacetamides, on the other hand, are rarely used because they are less electrophilic and more sterically demanding.
Soda Chanthamath, Seiji Iwasa, and colleagues, Toyohashi University of Technology, Japan, have developed the first highly stereoselective cyclopropanation of diazo Weinreb amides, a type of diazoacetamide, with olefins. The team used a chiral ruthenium phenyloxazoline complex as the catalyst, modified with a bulky quarternary ammonium group. They were able to synthesize a range of chiral cyclopropane amides (pictured) in dichloromethane at –30 °C.
The reactions had good to excellent yields (up to 99 %), diastereoselectivities of up to 99:1, and enantioselectivities up to 96 % ee. The resulting chiral Weinreb amides could be converted into useful synthetic intermediates such as alcohols, ketones, and aldehydes in one step.
- Highly Stereoselective Cyclopropanation of Diazo Weinreb Amides Catalyzed by Chiral Ru(II)-Amm-Pheox Complexes,
Soda Chanthamath, Hamada S. A. Mandour, Thu Thi Minh Tong, Kazutaka Shibatomi, Seiji Iwasa,
Chem. Commun. 2016.
DOI: 10.1039/C6CC02498J