Metal-air rechargeable batteries are half-open systems that consume oxygen from air. Especially zinc-air batteries have attracted attention, as they are environmentally friendly and safe. Unfortunately, zinc-air batteries still mainly rely on aqueous electrolytes, since alternative solid graphene oxide (GO) electrolyte membranes are mechanically very unstable.
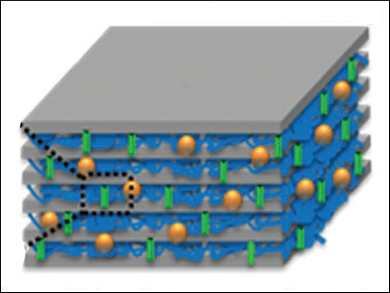
Zhongwei Chen, University of Waterloo, Ontario, Canada, and colleagues have developed a robust solid electrolyte for flexible zinc-air batteries using a laminated nanocellulose/GO membrane, functionalized with highly hydroxide-conductive quaternary ammonium (QA) groups. Functionalization was accomplished by the adsorbtion of dimethyloctadecyl [3-(trimethoxysilyl)-porpyl] ammonium chloride (DMAOP) to the various oxygen groups of cellulose and GO. The resulting QA-functionalized GO nanosheets (QAFGO) and cellulose fibers (QAFC) were vacuum filtered to form a laminate-structured membrane (QAFCGO, pictured).
The hydroxide-conducting QAFCGO was successfully applied in powering an electric fan, even under mechanical stress, such as bending the flexible membrane at an angle of 120°. Analysis of the hydroxide transport in QAFCGO revealed a complex process, where two different molecular hydroxide transport mechanisms, a Grotthuss proton-hopping mechanism and a vehicle mechanism involving water, take place in the two different layers simultaneously.
- Laminated Cross-Linked Nanocellulose/Graphene Oxide Electrolyte for Flexible Rechargeable Zinc-Air Batteries,
Jing Zhang, Jing Fu, Xueping Song, Gaopeng Jiang, Hadis Zarrin, Pan Xu, Kecheng Li, Aiping Yu, Zhongwei Chen,
Adv. Energy Mater. 2016.
DOI: 10.1002/aenm.201600476