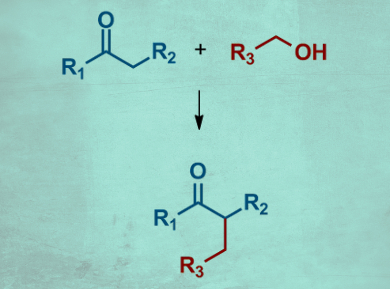
Borrowing hydrogen catalysis is an atom-economic green chemistry method to convert alcohols. A transition-metal catalyst “borrows” hydrogen from the alcohol, which is converted into an aldehyde and thus activated. The aldehyde can then react with a nucleophile, e.g., an enolate to form a C–C bond. The resulting alkene is reduced by the “borrowed” hydrogen to give the final product. The method is widely used for methyl ketones. The use of substituted ketones that give branched products (pictured), however, has not been extensively investigated.
Frank Glorius and colleagues, University of Münster, Germany, have developed an α-alkylation of methylene ketones with primary alcohols. The team used a ruthenium(II) complex with two N-heterocaclic carbene (NHC) ligands to catalyze the reaction of various ketones with a range of primary alcohols. The reaction was performed in tert-amyl alcohol (TAA)/n-hexane with two equivalents of LiOtBu as a base at 100 °C over 24 hours.
The reaction proceeded in good to excellent yields and tolerated functional groups such as aryl halides, cyanides, and heterocycles. However, functional groups that can be reduced, such as nitro groups or alkynes, proved problematic. The researchers demonstrated the synthetic potential by synthesizing the Alzheimer’s drug donepezil in one step. According to the team, the method ignificantly broadens the scope of borrowing hydrogen catalysis for alkylations.
- Ruthenium-NHC Catalyzed α-Alkylation of Methylene Ketones Provides Branched Products through Borrowing Hydrogen Strategy,
Christoph Schlepphorst, Biplab Maji, Frank Glorius,
ACS Catal. 2016.
DOI: 10.1021/acscatal.6b01351