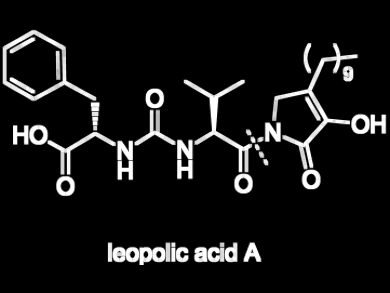
Sabrina Dallavalle and colleagues, Università degli Studi di Milano, Italy, report the first total synthesis of leopolic acid A, a fungal metabolite with antimicrobial activity and with a rare 2,3-pyrrolidinedione nucleus linked to an ureido dipeptide.
Crucial steps of the 11-step synthesis include a Dieckmann cyclization to obtain the 2,3-pyrrolidinedione ring and a Wittig olefination to install the polymethylene chain.
In adition, the team synthesized an oxazolidinone-containing leopolic acid A. Both compounds showed antibacterial activity.
 Total synthesis of leopolic acid A, a natural 2,3-pyrrolidinedione with antimicrobial activity,
Total synthesis of leopolic acid A, a natural 2,3-pyrrolidinedione with antimicrobial activity,
Atul A. Dhavan, Rahul D. Kaduskar, Loana Musso, Leonardo Scaglioni, Piera Anna Martino, Sabrina Dallavalle,
Beilstein J. Org. Chem. 2016, 12, 1624–1628.
DOI: 10.3762/bjoc.12.159