Nanoreactors can be used to catalyze reactions in a controlled manner. They usually consist of a catalytically active core and a permeable shell. The cores can be, e.g., nanoparticles or enzymes; shells have been made from porous silica, porous carbon, polymers, and other materials. Controlling the pore size of these shells, and thus the reactant transport, can be difficult. In contrast, the pore sizes of metal-organic frameworks (MOFs) can be tuned.
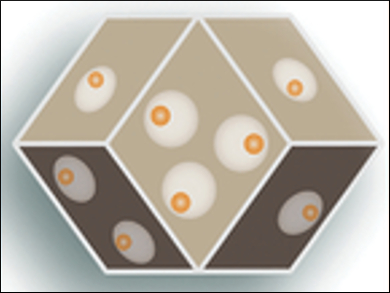
Dawei Wang and Cheng‐Yong Su, Sun Yat‐Sen University Guangzhou, China, and colleagues have developed a nanoreactor with a yolk-shell structure (pictured), using the single-crystalline MOF ZIF-8 as a shell. The team introduced macropores into the MOF and encapsulated one gold nanoparticle (Au NP) into each macropore to form a nanoreactor unit.
To produce this structure, the researchers coated Au NPs with silica. They then combined the coated NPs with ZIF-8 during crystallization, leading to an Au@silica@ZIF-8 nanostructure. In a final step, the silica was etched with NaOH, creating macropores around the nanoparticles. The number of reactor units in the MOF shell can be easily varied with this method.
The team tested the nanoreactor in the aerobic oxidation of different alcohols. The material has good molecular-size selectivity, conversion, and cycling stability. While 1-butanol and 1-hexanol are converted, the larger 3-phenylpropanol is not, since it cannot enter the MOF’s pores. The space around the NPs in the yolk-shell structure increases conversion compared to core-shell nanoreactors, where the NPs are encapsulated into the MOF directly. The researchers attribute this to improved reactant transfer and better accessibility of the nanoparticles’ catalytically active sites.
- Nanoreactor Based on Macroporous Single Crystals of Metal-Organic Framework,
Shuhai Wang, Yanan Fan, Jun Teng, Yan-Zhong Fan, Ji-Jun Jiang, Hai-Ping Wang, Hansjörg Grützmacher, Dawei Wang, Cheng-Yong Su,
Small 2016.
DOI: 10.1002/smll.201601873