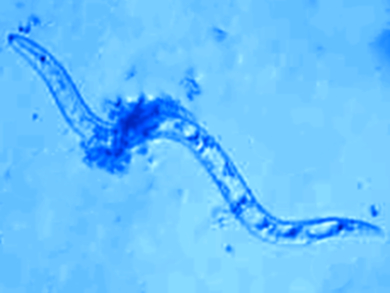
Parasitic nematodes, such as hookworms or heartworms, can cause diseases in both plants and animals. Drugs against such parasites are called antihelminthics. Their use is widespread, e.g., in pets, and as a result, some of the parasites have developed resistances against them. New antiparasitic drugs can overcome this temporarily, but the race against resistances is ongoing.
Robin B. Gasser, University of Melbourne, Parkville, Australia, Gilles Gasser, Université Pierre et Marie Curie, Paris, France, and colleagues have modified the drug monepantel, a member of a relatively new class of antiparasitics, to overcome these emerging resistances. Monepantel consists of an aryloxy group, a chiral spacer, and a benzamide unit. The team modified the compound by substituting the groups at either end of the molecule with metallocene groups (ferrocene or ruthenocene).
To prepare the ferrocene derivatives, the researchers reacted 2-amino-2-hydroxymethylproprionitrile with activated ferrocencarboxylic acid. The resulting intermediate was converted into a ferrocenyl-substituted monepantel analogue using a Williamson ether synthesis in the presence of NaH and 3-fluoro-4-(trifluoromethyl)benzonitrile. The team also prepared disubsituted analogues with two ferrocenyl groups, as well as ruthenocenyl analogues which are structurally identical to the ferrocenyl compounds.
The biological activity of the compounds was tested on the parasitic nematodes Haemonchus contortus (barber’s pole worm) and Trichostrongylus colubriformis. The ruthenocyl compounds were not active, but two of the ferrocenyl derivatives showed nematocidal activity. According to the team, the synthesized compounds could serve as leads for the development of new classes of anti-parasitic agents.
- Assessment of the Nematocidal Activity of Metallocenyl Analogues of Monepantel,
Jeannine Hess, Malay Patra, Abdul Jabbar, Vanessa Pierroz, Sandro Konatschnig, Spingler Bernhard, Stefano Ferrari, Robin Gasser, Gilles Gasser,
Dalton Trans. 2016.
DOI: 10.1039/c6dt03376h