Direct C-H bond activation is attractive from a step economy stand point, especially in the case of biaryl formation, as it avoids the need to preactivate two arenes. Many methods for direct oxidative cross-coupling have been reported, most based on a combination of palladium and a stoichiometric amount of copper or silver.
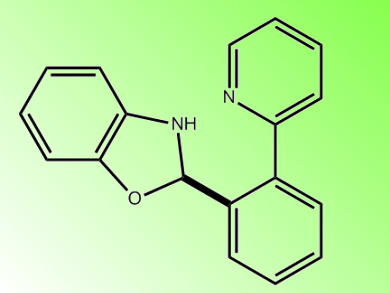
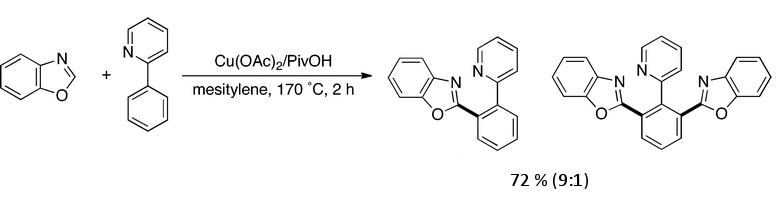
Masahiro Miura, Koji Hirano, and co-workers, Osaka University, Japan, report a direct biaryl coupling that proceeds under palladium-free conditions. They use Cu(OAc)2 and PivOH in mesitylene at 170 °C. The coupling of 2-phenylpyridine with benzoxazole was performed in 2 h to give the biaryl and 1:2 coupling product in 72 % combined yield. Full conversion could be achieved with greater than stoichiometric amounts of Cu and sterically hindered phenylpyridines such as 2-(2-methoxyphenyl)pyridine could also be coupled.
- Copper-Mediated Intermolecular Direct Biaryl Coupling
M. Kitahara, N. Umeda, K. Hirano, T. Satoh, M. Miura,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja111401h