Diaziridines and diazirines, which feature highly strained three-membered CN2 heterocycles, can be used as synthetic precursors in organic, biological, and medicinal chemistry. In contrast, group 13 species of the type EN2 (E = B, Al, Ga) that are isoelectronic with diazirines are less well explored—in spite of their potential as synthetic intermediates and building blocks.
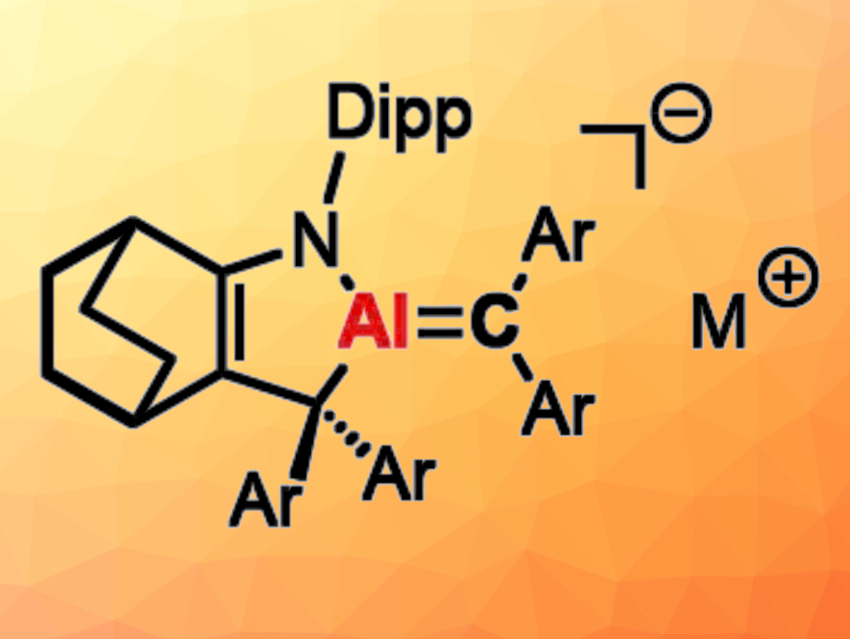
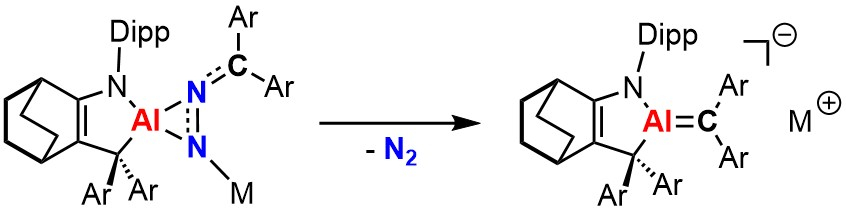
Chenting Yan and Rei Kinjo, Nanyang Technological University, Singapore have achieved the synthesis and isolation of an AlN2 three-membered ring species isoelectronic with diazirines (pictured below on the left). A [1+2] cycloaddition between a cyclic (alkyl)(amino)aluminyl anion and a diaryldiazomethane gives a thermally unstable AlN2 species (Dipp = 2,6-diisopropylphenyl, Ar = 3,5-di-tert-butylphenyl). At ambient temperature, the compound spontaneously releases N2 gas to give an ion-separated species featuring a short exocyclic Al–C bond (pictured below on the right). This bond has a pronounced Al=C π-bonding character, as was indicated by computational studies.
The Al=C moiety can undergo an intramolecular C−H activation at one of the isopropyl groups in the Dipp substituent. It can also react with diazo compounds, leading to a product with a tetracoordinate Al center substituted by two iminyl groups. This indicates a reduction and complete cleavage of the N=N double bond. Such a cleavage of terminal N=N double bonds by main group species is rare.
- A Three‐membered Diazo‐Aluminum Heterocycle to Access an Al=C π Bonding Species,
Chenting Yan, Rei Kinjo,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202211800