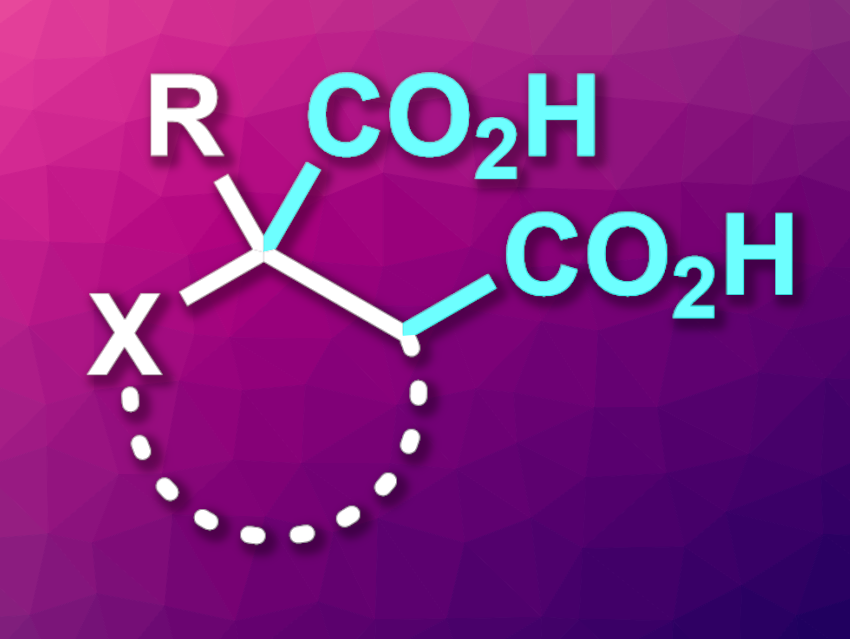
Succinic acid is a dicarboxylic acid of the type HOOC–(CH2)2–COOH. One approach to the synthesis of succinic acid derivatives is the dicarboxylation of alkenes (example product pictured). CO2 can be a useful C1 building block for carboxylation reactions. Dicarboxylations can be realized, e.g., electrochemically or photochemically.
Dong Guo, Xu Zhu, Xuzhou Medical University, China, and colleagues have developed a photocatalytic strategy for the synthesis of succinic acid derivatives via an alkene dicarboxylation using carbon dioxide and a formate salt as carbon sources. The team used 4CzIPN as a cyanoarene-based photocatalyst, Cs2CO3 as a base, and dimethyl sulfoxide (DMSO) as the solvent. A catalytic amount of 1,4-diazabicyclo[2.2.2]octane (DABCO) was used as a hydrogen atom transfer (HAT) reagent to abstract the hydrogen atom from formate. The reactions were performed under 1 atm of CO2 and blue LED irradiation.
The team converted different mono-, di-, and trisubstituted alkenes as well as acrylate, acrylamide, and indole derivatives into the corresponding diacids in moderate to good yields. According to the researchers, the reaction involves a reactive CO2•– intermediate, which acts as both a C1 source and a reductant. The products could have applications, e.g., in medicinal and polymer chemistry.
- Dicarboxylation of Alkenes with CO2 and Formate via Photoredox Catalysis,
Pei Xu, Sai Wang, Hui Xu, Yi-Qin Liu, Rui-Bo Li, Wen-Wen Liu, Xing-Yu Wang, Ming-Lin Zou, Yuan Zhou, Dong Guo, Xu Zhu,
ACS Catal. 2023, 13, 2149–2155.
https://doi.org/10.1021/acscatal.2c06377