Strong Lewis acids are useful for a variety of chemical reactions. However, their scalable use can be hampered by high costs and low convenience. Strongly acidic reagents can be created, e.g., by using carbon-based Lewis acids. Trityl cations are interesting in this context, but more acidic fluorinated examples are hard to isolate. Other electron-deficient systems such as iminium or pyridinium ions can also be used.
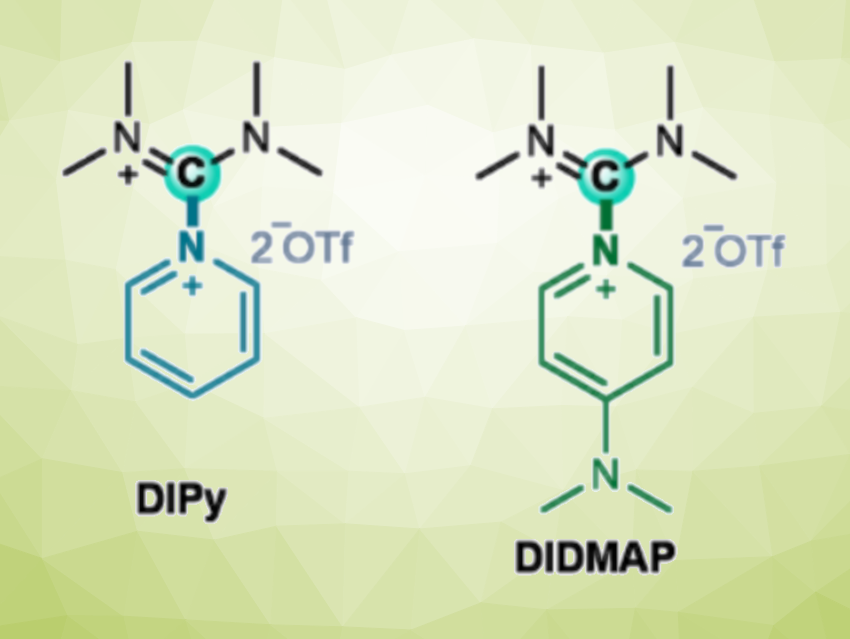
Florian F. Mulks, RWTH Aachen University, Germany, and colleagues have developed stable, convenient, carbon-based Lewis acids (examples pictured). The team prepared tetramethyldiiminium (DI) nucleophile adducts (“DINus”). They started from tetramethylurea, which was reacted with triflic anhydride (Tf2O) to form an isouronium salt (reactions pictured below). This salt was combined with pyridine (Py), which reacted smoothly via substitution at the carbon center to give the adduct DIPy. The product conveniently precipitated from dichloromethane (DCM). This synthesis was performed in a one-pot procedure at the 100 mmol scale (47 g), giving a yield of 98 %. The product is mildly hygroscopic but stable in air for several weeks.
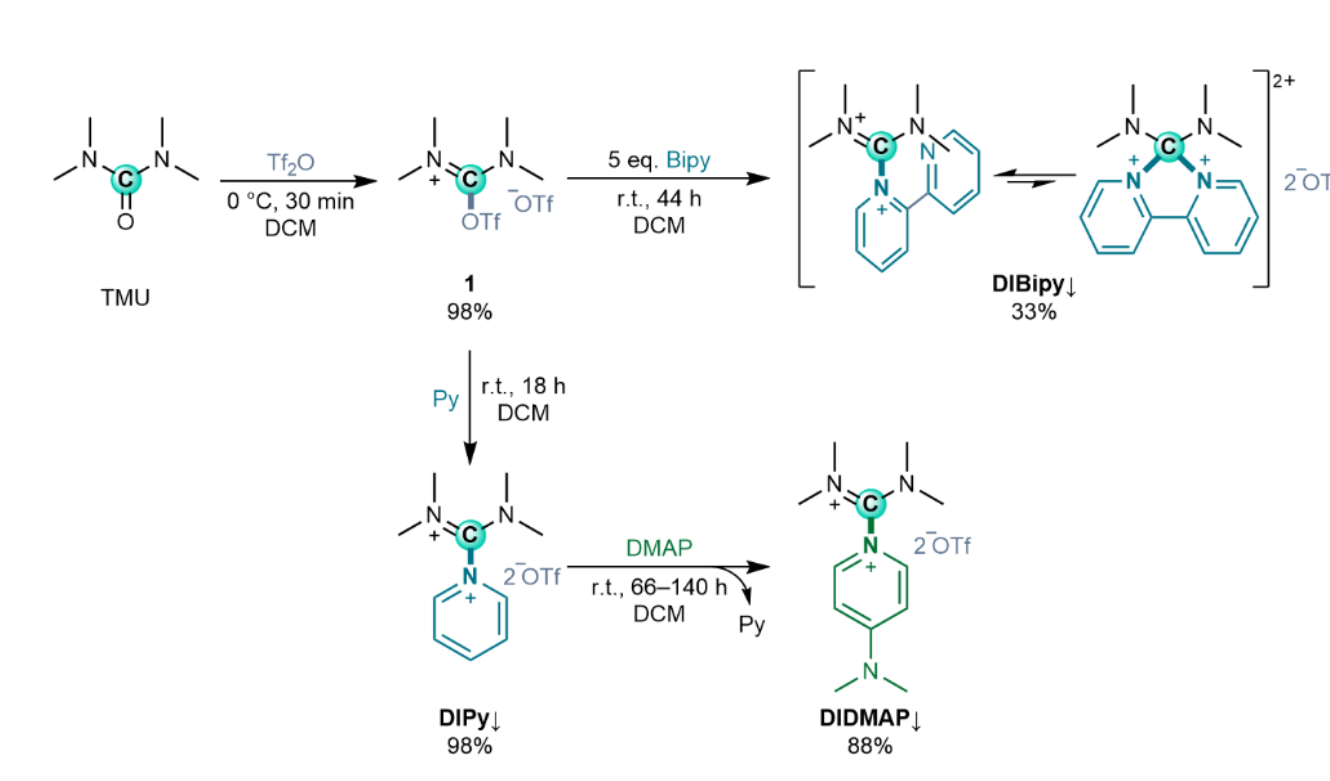
The team then substituted the pyridine in DIPy with the stronger donor DMAP (4-dimethylaminopyridine). The addition of DMAP to a suspension of DIPy in DCM gave the desired product (DIDMAP) in a yield of 88 %. An adduct with 2,2’-bipyridine (Bipy) was also synthesized, using an excess of Bipy to obtain the adduct DIBipy in 33 % yield.
The researchers found that the synthesized diiminium nucleophile adducts are convenient to prepare and handle and act as Lewis acids with strong fluoride, hydride, and oxide abstraction reactivity. The Lewis acids effectively produce acylpyridinium intermediates from carboxylates, which can acylate amines to give amides and imides. The team proposes that these reagents and their derivatives may be useful in a variety of applications.
- Diiminium Nucleophile Adducts are Stable and Convenient Strong Lewis Acids,
Niklas Bormann, Jas S. Ward, Ann Kathrin Bergmann, Paula Wenz, Kari Rissanen, Yiwei Gong, Wolf-Benedikt Hatz, Alexander Burbaum, Florian F. Mulks,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302089