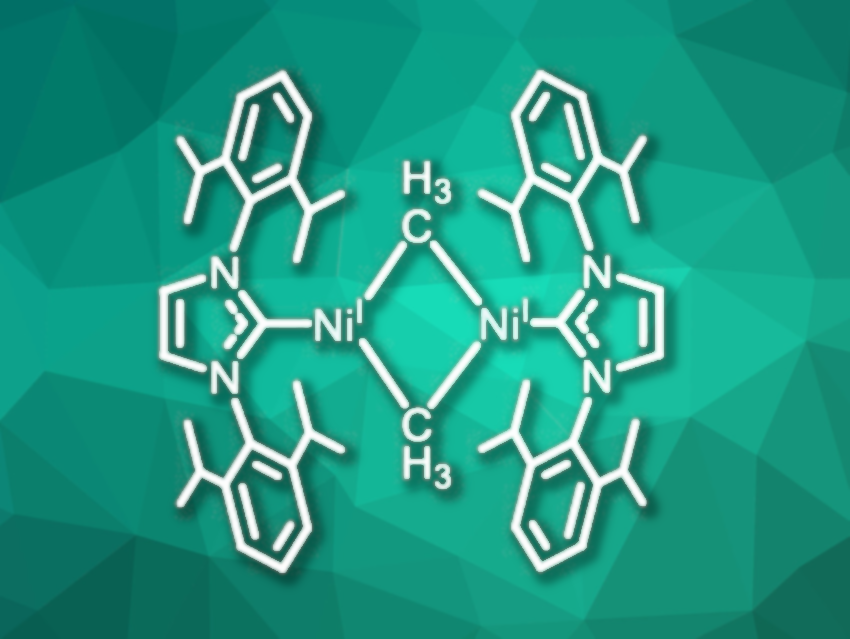
Nickel(I) complexes have been known for more than a century. They play important roles in various enzymes and can catalyze, e.g., the conversion of CO2 to CO and the formation of methane. In chemical synthesis, Ni(I) species are potential intermediates in Ni-catalyzed C–C cross-coupling reactions. This versatile catalytic activity makes Ni(I) species interesting synthetic targets.
T. Don Tilley and Ryan J. Witzke, University of California, Berkeley, USA, have synthesized a dimeric nickel(I)complex with two bridging methyl groups and two N-heterocyclic carbene (NHC) ligands (1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene or IPr, complex pictured). The complex was formed by a salt metathesis reaction from Sigman’s dimer [(IPr)Ni(μ-Cl)]2 and MeMgCl in diethylether at room temperature in 82 % yield. The diamagnetic complex was characterized by 1H NMR spectroscopy and X-ray diffraction analysis. Density functional theory (DFT) calculations indicate that the complex is diamagnetic due to antiferromagnetic coupling between unpaired spins at the two nickel centers.
The complex readily reacts with SiH4 to form an analogous complex with SiH3-type bridges under release of methane. The silyl complex is significantly more thermally stable than the methyl analogue, which decomposes upon heating and is reduced to nickel(0) under a hydrogen atmosphere. The complex can also activate O–H bonds: 2,6-di-tert-butyl-4-methylphenol (BHT) was successfully activated to give a phenoxy complex. The complex can also catalyze the coupling of aryl halides with methyl magnesium chloride at room temperature.
- Synthesis and Reactivity of a Dimeric Ni(I) Methyl Complex,
Ryan J. Witzke, T. Don Tilley,
Organometallics 2022.
https://doi.org/10.1021/acs.organomet.2c00188