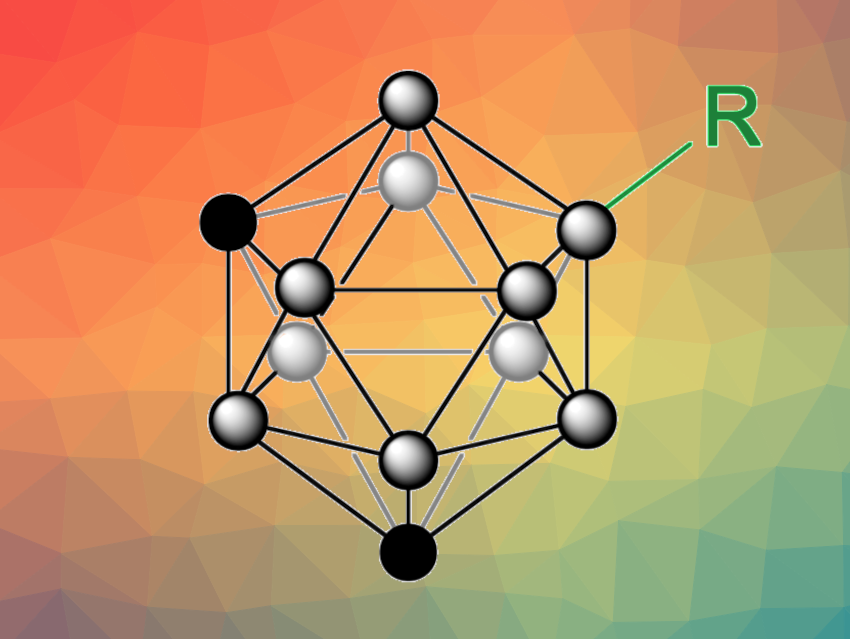
Radical-based reactions can be useful, e.g., in organoboron chemistry. However, for polyhedral boranes, such as icosahedral carboranes (C2B10H12), radical approaches to B–H functionalization are rare. B–H functionalizations of carboranes can be challenging due to the high B–H bond dissociation energies, and selective transformations can be difficult due to the similar chemical environments of the ten B–H bonds.
Deshuang Tu, Chang-Sheng Lu, Hong Yan, Nanjing University, China, and colleagues have developed a method for the direct B–H functionalization of icosahedral carboranes via B–H homolysis. The transformation is based on a nitrogen-centered radical-mediated hydrogen atom transfer (HAT) strategy. The team reacted different m-carboranes, o-carboranes, and p-carboranes with nitrogen-centered radical (NCR) precursors under blue LED light to generate boron-centered carboranyl radicals, using PhCF3 as the solvent. These radicals can react with different radical traps (or “SOMO-philes”, SOMO = singly occupied molecular orbital) to achieve functionalization of the carborane.
Using this approach, the team performed thiolations, selenations, alkynylations, alkenylations, cyanations, and halogenations. The selectivity of the reaction is based on the preference of the HAT process to take place at the B–H bond with the lowest bond dissociation energy. For m-carborane, for example, the B(9)–H bond has the lowest dissociation energy with 99.4 kcal/mol according to density functional theory (DFT) calculations and is preferentially functionalized. Overall, the work provides a new approach to the straightforward B–H activation and functionalization of carboranes.
- Direct B–H Functionalization of Icosahedral Carboranes via Hydrogen Atom Transfer,
Hongyuan Ren, Ping Zhang, Jingkai Xu, Wenli Ma, Deshuang Tu, Chang-sheng Lu, Hong Yan,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c01314