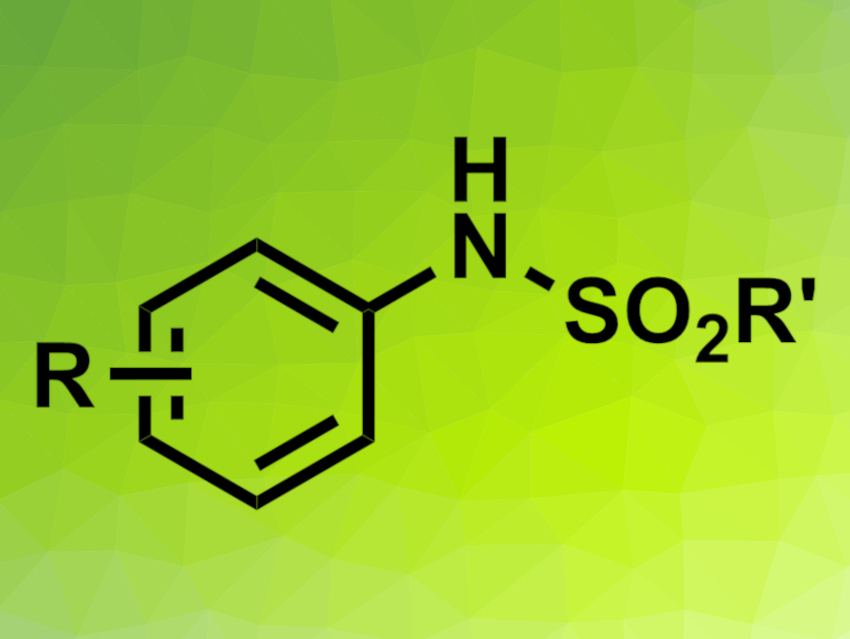
Sulfonamides are often present, e.g., in biologically active compounds. They can be synthesized from amines and sulfonyl chlorides, which are, in turn, obtained from nitro compounds and thiols. Avoiding these extra steps and directly reacting nitro compounds with thiols would be useful to improve the synthesis of sulfonamides.
Shi-Chao Ren, Yonggui Robin Chi, Guizhou University, Guiyang, China, and Nanyang Technological University, Singapore, and colleagues have developed a strategy for the direct coupling of nitro compounds and thiols to form sulfonamides using photoexcitation. The team reacted a variety of nitroarenes with different thiols in acetone as the solvent under blue LED light or UV light at 0 °C. The reaction does not need a photocatalyst.
The desired sulfonamides were obtained in mostly moderate to good yields. The team proposes that the reaction starts with the photoexcitation of the nitroarene to its electronically excited state, which can act as an oxidant. This oxidant reacts with the thiol molecules and is converted to a nitrosoarene. The nitrosoarene can then be converted to the desired sulfonamide. Overall, oxygen atoms from the nitro compound are transferred to the thiol’s sulfur atom.
The researchers caution that further improvements are needed. Still, they point out that the developed strategy could lead to a clean method for sulfonamide synthesis from nitro compounds and thiols.
- Direct Reaction of Nitroarenes and Thiols via Photodriven Oxygen Atom Transfer for Access to Sulfonamides,
Zhaowei Bao, Juan Zou, Chengli Mou, Zhichao Jin, Shi-Chao Ren, Yonggui Robin Chi,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c03770