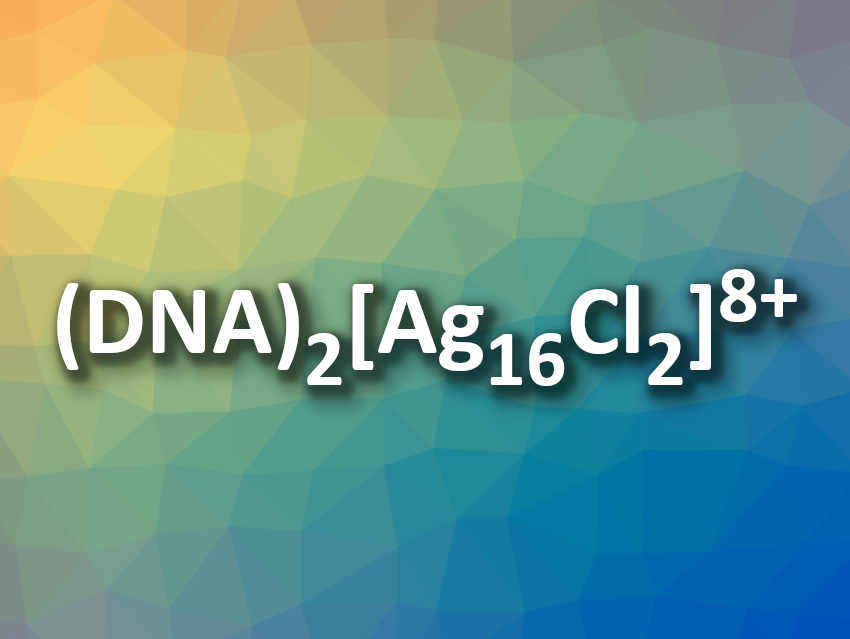DNA-stabilized silver nanoclusters can act as emitters and might have applications, e.g., in imaging and sensing. Their properties can be tuned by changing the DNA sequences. Nanoclusters that are stabilized by DNA can be more challenging to study than nanoclusters that are protected by a layer of small molecules. Nevertheless, high-performance liquid chromatography (HPLC) can be used to isolate specific DNA-stabilized silver nanocluster species, and high-resolution electrospray ionization mass spectrometry (ESI-MS) can then be used to determine the mass, charge, and effective valence electron count of the clusters. There also are crystal structures available for some DNA-stabilized silver nanoclusters.
Stacy M. Copp, University of California, Irvine, USA, and colleagues have investigated rod-like DNA-stabilized silver nanoclusters with 16 silver atoms that emit in the near-infrared (NIR) range, aiming to determine their effective valence electron count. The team performed ESI-MS measurements and found that the detected mass did not correspond to a cluster made up of only silver atoms and the employed DNA strands. Instead, the nanoclusters have the composition (DNA)2[Ag16Cl2]8+. According to researchers, this is the first evidence of DNA-stabilized silver nanoclusters with additional chloride ligands.
The team performed a ligand exchange of chloride with bromide using NaBr, which gave (DNA)2[Ag16ClBr]8+ and (DNA)2[Ag16Br2]8+ and confirmed the halide nature of the ligands. They also observed that (DNA)2[Ag16Cl2]8+ is unusually stable. Thus, developing silver nanoclusters with mixed DNA and chloride ligands could be useful for practical applications.
- Chloride Ligands on DNA-Stabilized Silver Nanoclusters,
Anna Gonzàlez-Rosell, Sami Malola, Rweetuparna Guha, Nery R. Arevalos, María Francisca Matus, Meghen E. Goulet, Esa Haapaniemi, Benjamin B. Katz, Tom Vosch, Jiro Kondo, Hannu Häkkinen, Stacy M. Copp,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c01366