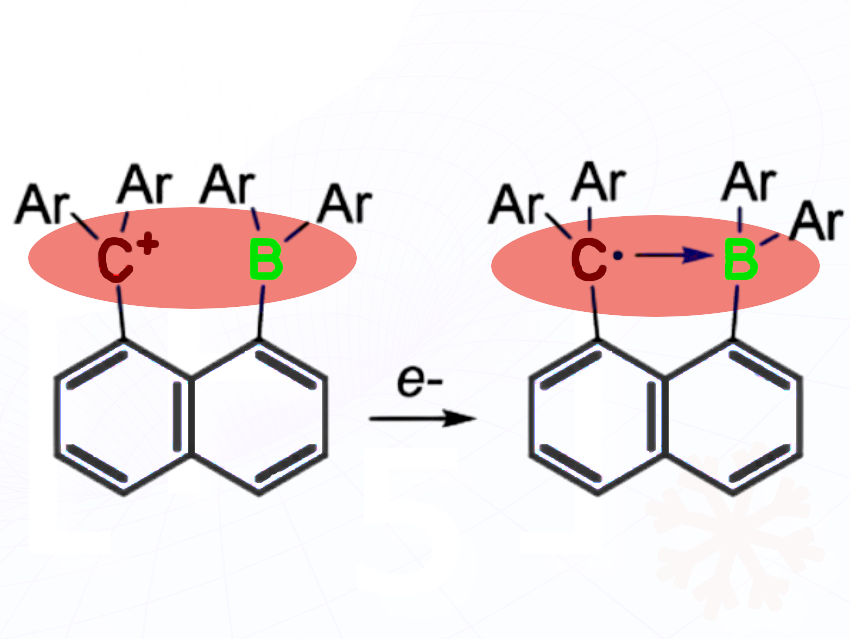
François P. Gabbaï and colleagues, Texas A&M University, College Station, USA, have built a rigid naphthalene-based molecule with a boron and a carbon unit positioned close together to test whether a one-electron B–C bond could exist. They reduced the system stepwise and studied its structure and electronic properties using spectroscopy and crystallography.
The researchers found that the single electron mostly resides on the carbon, creating a weak, noncovalent triel interaction rather than a true covalent bond. Injecting a second electron forms a normal two-electron B–C bond. This demonstrates the difficulty of forming heteronuclear one-electron σ bonds.
According to the researchers, their study clarifies how electrons distribute in mixed-element radicals, guiding design of new radical-based reactions and materials. It also highlights fundamental limits of single-electron bonding between different elements.
- One-Electron Boron–Carbon Triel Bonding
Paula Castro CastroWei-Chun LiuFrançois P. Gabbaï,
J. Am. Chem. Soc. 2025
https://doi.org/10.1021/jacs.5c13589