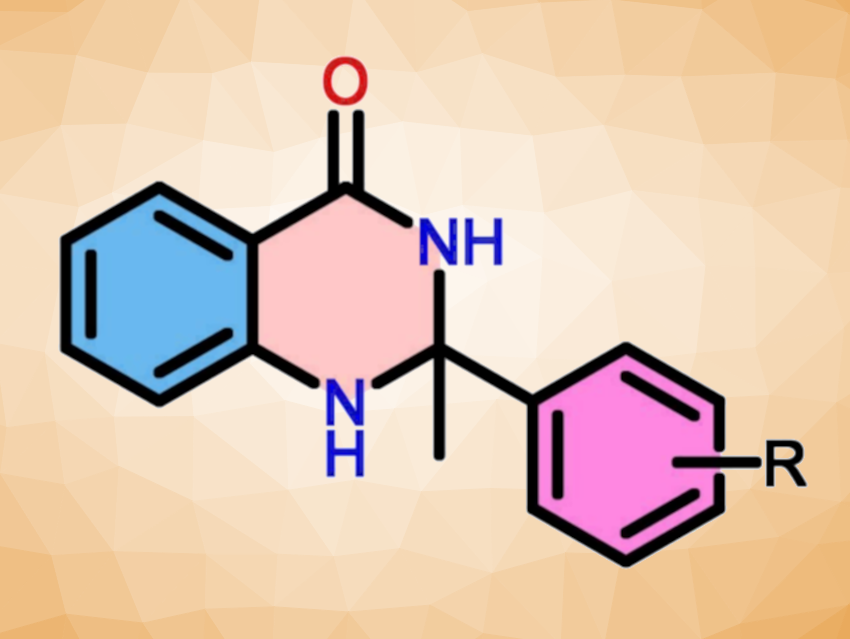
Techniques for the preparation of N-heterocyclic compounds are interesting research goals. Dihydroquinazolinone derivatives, for example, are useful substructures in, e.g., medicinal chemistry. They can be prepared via a formal Markownikoff’s double hydroamination of alkynes. However, this type of reaction usually requires homogeneous gold or platinum catalysts.
Arun Kumar Macharla, CSIR-Indian Institute of Chemical Technology, Hyderabad, India, and University of Hyderabad, Narender Nama, CSIR-Indian Institute of Chemical Technology and Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India, and colleagues have developed a method for the preparation of dihydroquinozolinones using a heterogeneous catalyst. The team used a beta (β)-zeolite-supported CuO catalyst to synthesize 2,3-dihydroquinazolin-4(1H)-ones (pictured above) via the double hydroamination of alkynes.
The team first prepared the zeolite HBeta (Hβ) via the calcination of commercially available ammonium beta zeolite (NH4β). The product was added to a solution of Cu(NO3)2·3H2O in water and heated, dried, and calcined to obtain the desired catalyst (Cuβ). The hydroamination of alkynes (pictured below) was then performed using Cuβ under solvent-free conditions at 130 °C.
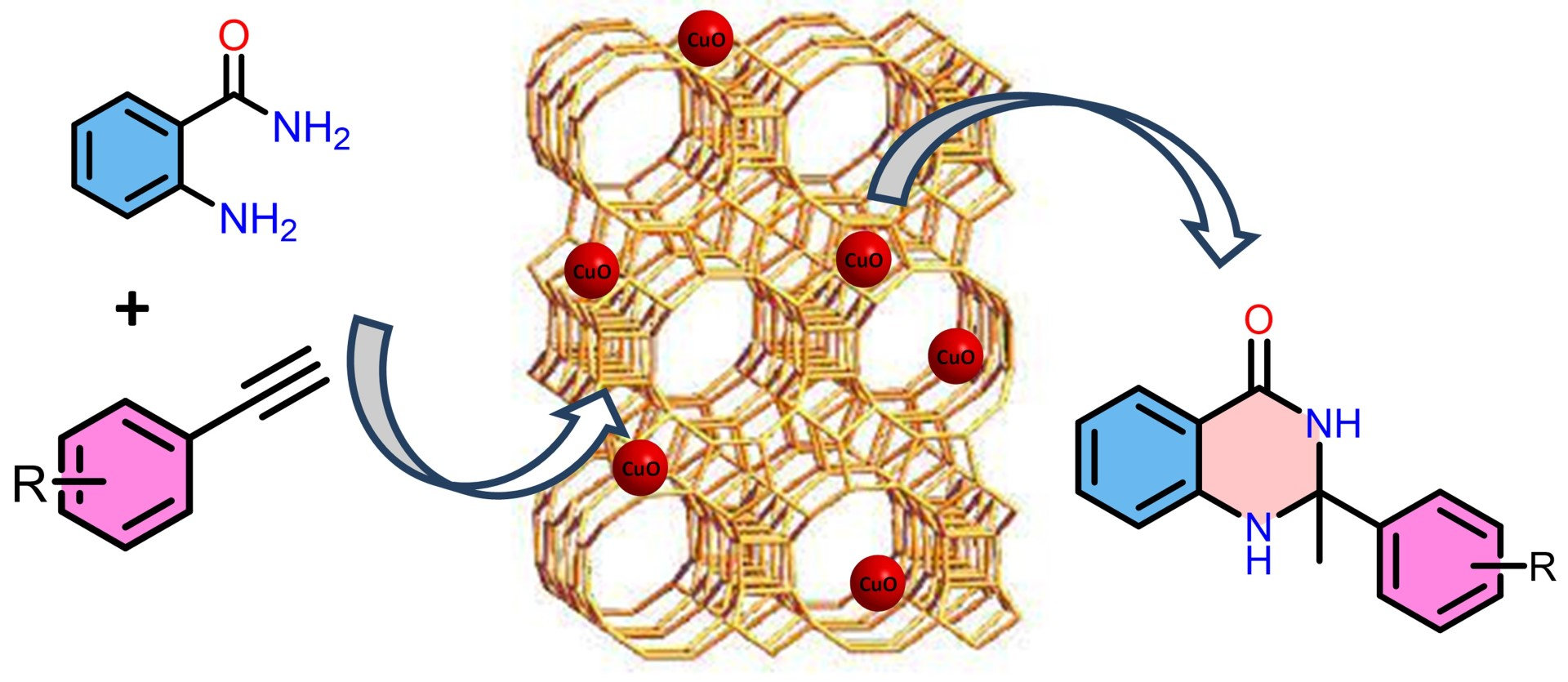
The desired 2,3-dihydroquinazolin-4(1H)-ones were obtained in moderate to excellent yields. A wide variety of functionalized phenyl acetylenes was converted, with both electron-rich and electron-deficient substituents. The catalyst was successfully reused for up to five consecutive cycles.
- Double Hydroamination of Terminal Alkynes over Beta Zeolite‐Supported CuO Catalyst,
Krishna Sai Gajula, Arun Kumar Macharla, Vasu Amrutham, Divya Rohini Yennamaneni, Murali Boosa, Ramulamma Madasu, Anil Kumar Chelukalapally, Narender Nama,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202300831