Cyclobutadiene is very reactive and tends to dimerize. This hampers its direct use in organic reactions such as [4 + 2] cycloadditions. However, reagents that release cyclobutadiene can allow such transformations. A cyclobutadiene iron tricarbonyl complex, for example, can release cyclobutadiene. However, the synthesis of this complex can involve toxic and/or sensitive reagents, and the release of cyclobutadiene can require harmful heavy metal oxidants.
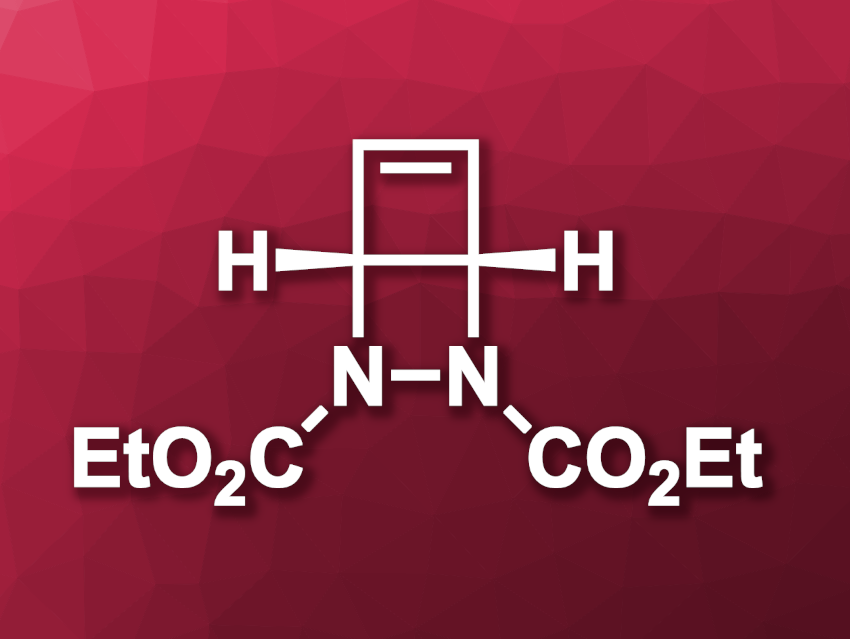
Noah Z. Burns, Stanford University, CA, USA, and colleagues have developed an alternative, easily accessible reagent that can release cyclobutadiene under mild conditions, i.e., diethyldiazabicyclohexene dicarboxylate (pictured). The team developed a scalable two-step synthesis of this compound starting from 2-pyrone, which was reacted with diethylazodicarboxylate to give a dihydropyridazine derivative. This intermediate was irradiated with light (350 nm) to induce a ring-closing and give the desired product.
The obtained reagent can be hydrolyzed, followed by a two-electron oxidation and double decarboxylation to give diaza-Dewar benzene (a compound with two fused four-membered rings that features two adjacent nitrogen atoms). The team used potassium hydroxide and (diacetoxyiodo)benzene for these steps. Upon nitrogen loss, cyclobutadiene is released and can be used in intermolecular [4 + 2] reactions with different electron-deficient alkenes.
- A Metal-Free Cyclobutadiene Reagent for Intermolecular [4 + 2] Cycloadditions,
Benjamin R. Boswell, Carl M. F. Mansson, Gabrielle E. Cabrera, Calvin R. Hansen, Allen G. Oliver, Noah Z. Burns,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c01591