Formamides are widely used chemicals, e.g., as solvents and reagents as well as in lithium-ion batteries. Their synthesis from amines and CO2 is a promising approach that involves the conversion of CO2 into value-added products. However, efficient catalytic systems for this reaction are rare. The transformations often require harsh reaction conditions, noble-metal catalysts, and base additives.
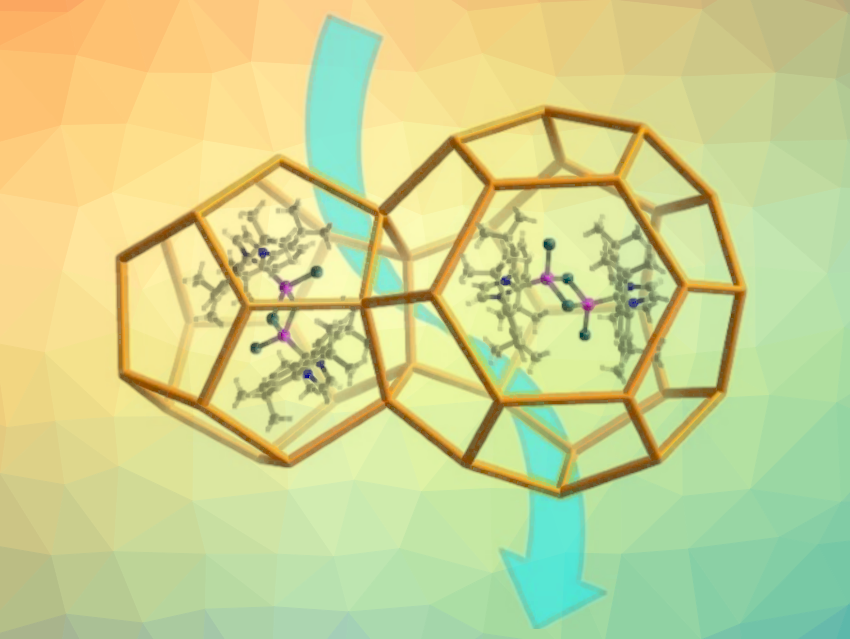
Jian-Gong Ma, Xiao Liu, Nankai University, Tianjin, China, and colleagues have used a metal–organic framework (MOF) as both a support and a micro-reactor to encapsulate a binuclear N-heterocyclic carbene cobalt complex, [Co(IPr)Br]2(μ-Br)2 (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, encapsulation pictured below). According to the researchers, this is the first example of host–guest composites featuring a MOF-encapsulated binuclear NHC complex.
The team used MIL-101(Cr) as the MOF support. The NHC ligand can enter the MOF cage and then form the desired binuclear cobalt complex upon addition of a cobalt salt. The resulting complex is trapped in the MOF.
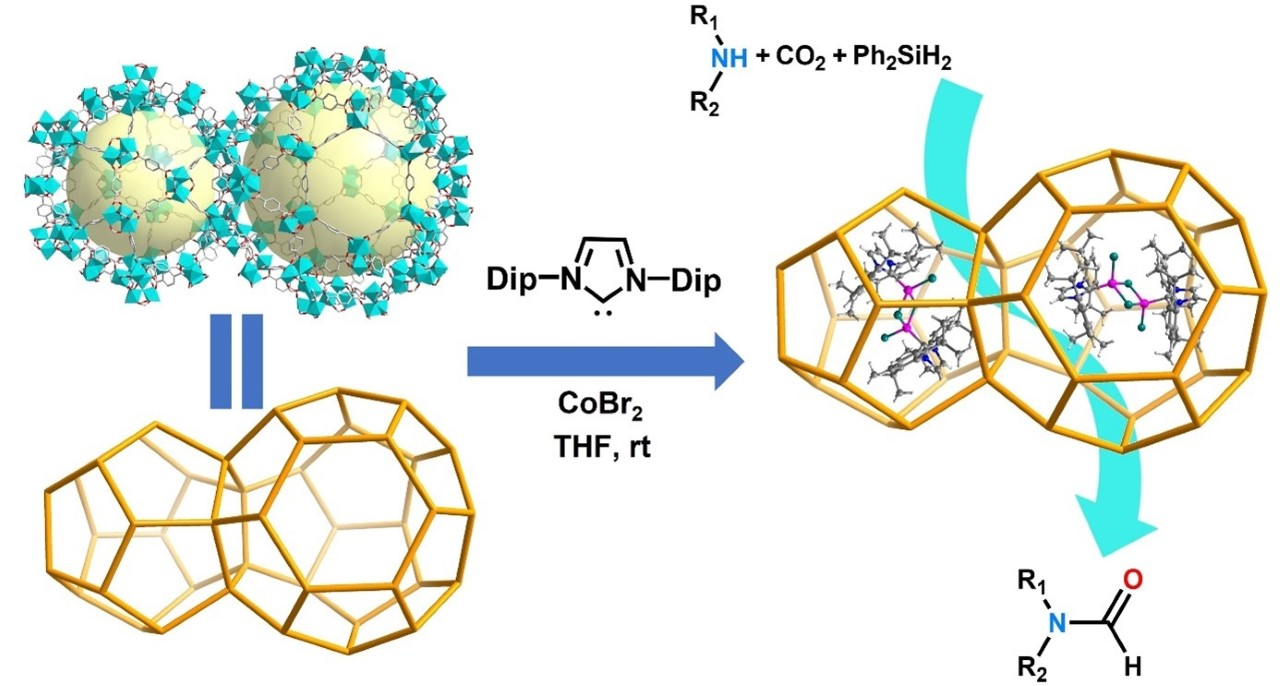
The noble-metal-free encapsulated catalyst combines some of the advantages of homo- and heterogeneous catalysis. It catalyzes the synthesis of various formamides from CO2 with amines and silanes (pictured above on the right) without any other additives. The catalyst shows promising stability, activity, efficiency, reusability, and substrate adaptability, and could contribute to CO2 management and the environmentally friendly production of formamides.
- MOF‐Incorporated Binuclear N‐Heterocyclic Carbene‐Cobalt Catalyst for Efficient Conversion of CO2 to Formamides,
Zhenzhen Zhou, Xiao Liu, Jian‐Gong Ma, Peng Cheng,
ChemSusChem 2022.
https://doi.org/10.1002/cssc.202201386