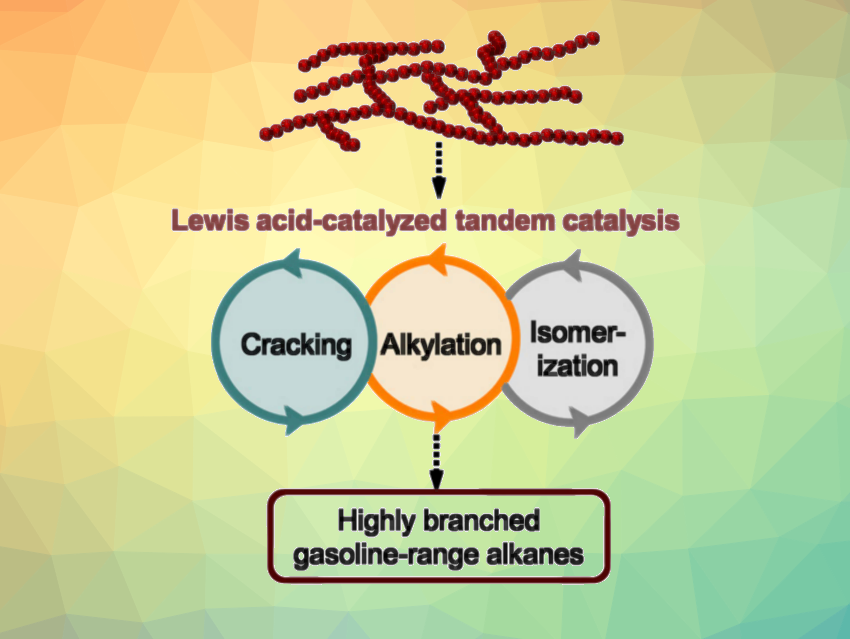
The large amounts of polymer waste produced today create environmental problems due to their resistance to degradation. Reusing polyolefins such as polyethylene (PE) and polypropylene (PP) as hydrocarbon feedstocks would be useful in this context. However, the C–C bonds in these polymers can be challenging to break, which makes their upcycling difficult. Thermal and catalyst-aided cracking reactions usually require a high energy input and provide insufficient control over the product distribution, producing low-value hydrocarbon mixtures. A tandem cracking-alkylation strategy could be an alternative to transform polyolefins into liquid alkanes that can be used as fuels.
Johannes A. Lercher, Pacific Northwest National Laboratory (PNNL), Richland, WA, USA, and Technical University of Munich (TUM), Garching, Germany, and colleagues have developed a method for the low-temperature deconstruction of polyolefins, using isopentane as an alkylating agent, Lewis acids as catalysts, and dichloromethane (DCM) as a solvent. The team investigated a wide range of Lewis acidic chlorides and found that anhydrous aluminum chloride (AlCl3) and gallium chloride (GaCl3) can achieve complete conversion of low-density polyethylene (LDPE) at 60 °C, with selectivities for gasoline-range liquid alkanes of over 70 %.
To better understand the process, the researchers performed kinetic and mechanistic studies using different Lewis acids. They found that the rates of LDPE conversion do not correlate directly with the strength of the Lewis acids. Instead, both AlCl3 and GaCl3 form a donor-acceptor complex with DCM, which can lead to a carbenium ion/MCl4– ion pair and initiate the carbenium-ion chemistry necessary for the desired transformation. This is followed by intermolecular hydride transfers, isomerizations, and C−C bond cleavage in the polyolefin. Overall, the polar environment created by the Lewis acids stabilizes carbenium ions and catalyzes the hydride transfer, allowing efficient LDPE deconstruction.
- Chloride and hydride transfer as keys to catalytic upcycling of polyethylene into liquid alkanes,
Wei Zhang, Hai Yao, Rachit Khare, Peiran Zhang, Boda Yang, Wenda Hu, Debmalya Ray, Jianzhi Hu, Donald M. Camaioni, Huamin Wang, Sungmin Kim, Mal-Soon Lee, Michele L. Sarazen, Jingguang G. Chen, Johannes Lercher,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202319580