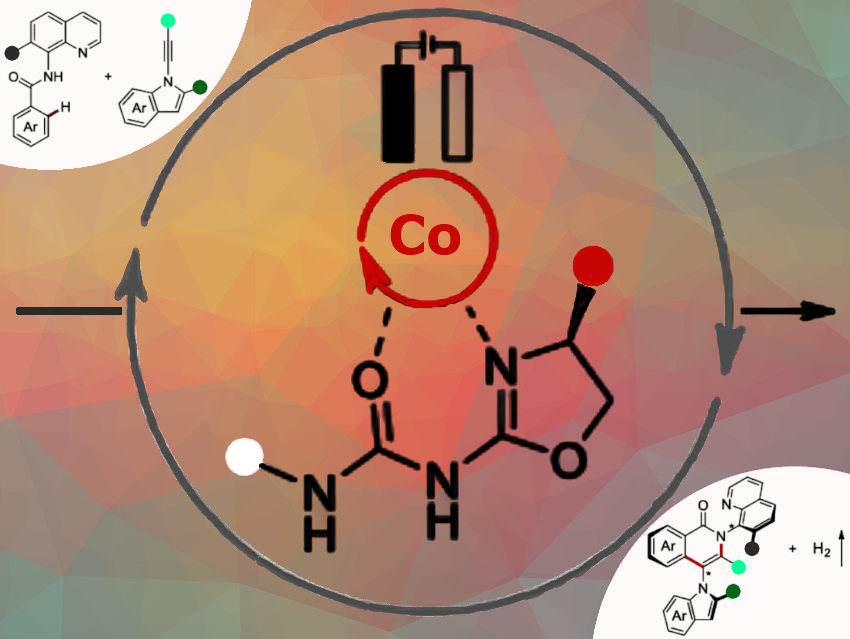
Enantioselective catalysis is a key approach in organic synthesis for producing chiral bioactive compounds and advanced materials. Lutz Ackermann, University of Göttingen, Germany, and coauthors have introduced a new class of chiral oxazoline ureas as κ²-N,O-preligands for enantioselective transition metal catalysis, addressing the ongoing need for tunable, efficient ligands to support greener and more diverse synthesis.
What did you do?
We devised a novel electrocatalytic strategy using cobalt and newly designed chiral oxazoline urea ligands to construct molecules with one or two chiral C–N axes. By selective C–H and N–H activation/annulation with 1-alkynyl indoles, the efficient synthesis of complex, chiral indole-based scaffolds is enabled. The reaction proceeds under mild electrochemical conditions and produces hydrogen gas as a valuable byproduct. The disclosed approach features a broad applicability and delivers high enantio- and diastereoselectivity.
To rationalize selectivity and explore alternative pathways, we employed principal component analysis (PCA), multivariate linear regression (MVLR) modeling, and density functional theory (DFT) calculations.
Why are you interested in this?
Chiral C–N axial compounds are essential in pharmaceuticals and materials but are challenging to synthesize selectively and in a sustainable fashion. Existing approaches commonly require precious metals or complex ligands and show limited substrate diversity. We aimed to create a more resource-economic, versatile, and efficient strategy using earth-abundant cobalt and electrochemistry. Hence, our goal was to advance enantioselective synthesis by unlocking access to valuable chiral structures, while reducing waste and energy consumption.
What is new and cool about this work?
We introduced a new class of chiral oxazoline urea ligands featuring unique performance in enantioselective cobalta-electrocatalysis. Our strategy enables dual axial chirality formation with high selectivity under mild conditions, furnishing only hydrogen gas as a byproduct.
Our integration of MVLR modeling to rationalize structure–enantioselectivity relationships between ligand and substrate components is particularly innovative. Given the multiple potential coordination modes of these ligands, key mechanistic pathways were thoroughly analyzed via DFT.
What are your key findings?
We showed that oxazoline urea ligands are highly effective in cobalt-catalyzed enantioselective electrosynthesis. The approach provides access to complex, C–N axially chiral molecules in high yield and selectivity, including molecules with two chiral axes. Electrolysis is essential for the reaction, and the ligand structure plays a key role in determining selectivity.
Our data-driven modeling enables performance prediction and mechanistic insight, broadening the toolkit for sustainable enantioselective catalysis.
What is the longer-term vision for your research?
These axially chiral indoles could serve as building blocks for pharmaceuticals, agrochemicals, and chiral ligands in other catalytic processes. The approach might be adapted to synthesize a wider variety of chiral heterocycles or natural product analogs. We also hope to expand the ligand platform to other transition metals and transformations.
The ultimate goal is to develop general, modular tools for sustainable enantioselective catalysis.
What part of your work was the most challenging?
Developing effective ligands that provided both good yields and high enantioselectivity was very challenging. Many ligand candidates failed or showed only modest selectivity, and fine-tuning their structures required careful design and evaluation.
Additionally, optimizing electrochemical conditions while ensuring catalyst stability increased complexity.
Mechanistic understanding through computational studies was also demanding, especially during the extraction of meaningful descriptors for both the preligand and coordinated states, as well as accurately modeling solvent mixtures in detailed DFT analyses. Striking the right balance between experimental observations and theoretical predictions was essential, yet not always straightforward.
Anything else you would like to add?
This work underscores the power of integrating synthetic innovation in 3d-metallaelectro-catalyzed C─H activations with data-driven design and computational chemistry. Our study emphasizes sustainability without compromising performance, and we hope it inspires further advancements in the development of enantioselective electrocatalysis.
The modularity of our ligand design also holds promise for broad application across diverse catalytic manifolds.
Thank you very much for sharing these insights.
The paper they talked about:
- Multiple Atropo Selectivity by κ²-N,O-Oxazoline Urea Ligands in Cobaltaelectro-Catalyzed C–H Activations: Decoding Selectivity with Data Science Integration,
Philipp Boos, Neeraj Kumar Pandit, Suman Dana, Tristan von Münchow, Airu Hashidoko, Laura Haberstock, Regine Herbst-Irmer, Dietmar Stalke, Lutz Ackermann,
ChemistryEurope 2025.
https://doi.org/10.1002/ceur.202500071
Lutz Ackermann is a Professor at the Institute for Organic and Biomolecular Chemistry and Director of the Wöhler Research Institute for Sustainable Chemistry at the Georg-August-Universität Göttingen in Germany.